Abstract
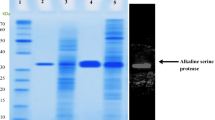
A gene that codes for an alkaline phosphatase was cloned from the thermophilic bacterium Meiothermus ruber, and its nucleotide sequence was determined. The deduced amino acid sequence indicates that the enzyme precursor including the putative signal sequence is composed of 503 amino acid residues and has an estimated molecular mass of 54,229 Da. Comparison of the peptide sequence with that of the prototype alkaline phosphatase from Escherichia coli revealed conservation of the regions in the vicinity of the corresponding phosphorylation site and metal binding sites. The protein was expressed in E. coli and its enzymatic properties were characterized. In the absence of exogenously added metal ions, activity was negligible; to obtain maximal activity, addition of free Mg2+ ions was required. Zn2+ ions had an inhibitory effect on the activity of the M. ruber enzyme. The pH and temperature optima for activity were found to be 11.0 and 62°C, respectively. The enzyme was moderately thermostable: it retained about 50% activity after incubation for 6 h at 60°C, whereas at 80°C it was completely inactivated within 2 h. The Michaelis constant for cleavage of 4-nitrophenylphosphate was 0.055 mM. While having much in common with other alkaline phosphatases, the M. ruber enzyme presents some unique features, such as a very narrow pH range for activity and an absolute requirement for magnesium for activity.




Similar content being viewed by others
References
Becker G, Hengge-Aronis R (2001) What makes an Escherichia coli promoter σs dependent? Role of the −13/−14 nucleotide promoter positions and region 2.5 of σs. Mol Microbiol 39:1153–1165
Bradshaw RA, Cancedda F, Ericsson LH, Neumann PA, Piccoli SP, Schlesinger MJ, Shriefer K, Walsh KA (1981) Amino acid sequence of Escherichia coli alkaline phosphatase. Proc Nat Acad Sci USA 78:3473–3477
Chang CN, Kuang W-J, Chen EY (1986) Nucleotide sequence of the alkaline phosphatase gene of Escherichia coli. Gene 44:121–125
Dong G, Zeikus JG (1997) Purification and characterization of alkaline phosphatase from Thermotoga neapolitana. Enzyme Microb Technol 21:335–340
Egorova LA, Loginova LG (1984) Selection of a culture forming alkaline phosphatase from thermophilic bacteria in genus Thermus (in Russian). Microbiologiia 53:242–245
Fernley HN (1971) Mammalian alkaline phosphatases. In: Boyer PD (ed) The Enzymes, vol 4. Academic Press, New York, pp 417–447
Ferreira AM, Wait R, Nobre MF, Costa MS (1999) Characterization of glycolipids from Meiothermus spp. Microbiology 145:1191–1199
Jablonski E, Moomaw EW, Tullis RH, Ruth JL (1986) Preparation of oligodeoxynucleotide-alkaline phosphatase conjugates and their use as hybridization probes. Nucleic Acids Res 14:6115–6128
Janeway CML, Xu X, Murphy JE, Chaidaroglou A, Kantrowitz ER (1993) Magnesium in the active site of Escherichia coli alkaline phosphatase is important for both structural stabilization and catalysis. Biochemistry 32:1601–1609
Ji CN, Jiang T, Chen MQ, Sheng XY, Mao YM (2001) Purification, crystallization and preliminary X-ray studies of thermostable alkaline phosphatase from Thermus sp. 3041. Acta Cryst D57:614–615
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Hulett FM, Kim EC, Bookstein C, Kapp NV, Edwards CW, Wyckoff HW (1991) Bacillus subtilis alkaline phosphatases III and IV. J Biol Chem 266:1077–1084
Kim EE, Wyckoff HW (1989) Structure of alkaline phosphatases. Clin Chim Acta 186:175–187
Kim EE, Wyckoff HW (1991) Reaction mechanism of alkaline phosphatase based on crystal structures. Two-metal ion catalysis. J Mol Biol 218:449–464
Kim YJ, Park TS, Kim HK, Kwon ST (1997) Purification and characterization of a thermostable alkaline phosphatase produced by Thermus caldophilus GK24. J Biochem Mol Biol 30:262–268
Kreil G (1981) Transfer of proteins across membranes. Annu Rev Biochem 50:317–348
Krupianko VI, Kolot MN, Nesmeianova MA (1981) Comparative study of properties of periplasmic and membrane-bound alkaline phosphatase of E. coli (in Russian). Biokhimiia 46:1249–1257
Laemmli UK (1970) Cleavage of the structural proteins during the assembly of the head of the bacteriophage T4. Nature 227:680–685
Manson MM (ed) (1992) Immunochemical protocols. Humana Press, Totowa, N.J., pp 480
Marmur J (1961) A procedure for the isolation of deoxyribonucleic acid from microorganisms. J Mol Biol 3:208–218
Mori S, Okamoto M, Nishibori M, Ichimura M, Sakiyama J, Endo H (1999) Purification and characterization of alkaline phosphatase from Bacillus stearothermophilus. Biotechnol Appl Biochem 29:235–239
Murphy JE, Xu X, Kantrowitz ER (1993) Conversion of a magnesium binding site into a zinc binding site by a single amino acid substitution in Escherichia coli alkaline phosphatase. J Biol Chem 268:21497–21500
Murphy JE, Tibbitts TT, Kantrowitz ER (1995) Mutation at positions 153 and 328 in Escherichia coli alkaline phosphatase provide insight towards the structure and function of mammalian and yeast alkaline phosphatases. J Mol Biol 253:604–617
Nobre MF, Trüper HG, Costa MS (1996) Transfer of Thermus ruber (Loginova et al. 1984), Thermus silvanus (Tenreiro et al. 1995) and Thermus chliarophilus (Tenreiro et al. 1995) to Meiothermus gen. nov. as Meiothermus ruber comb. nov., Meiothermus silvanus comb. nov. and Meiothermus chliarophilus comb. nov., respectively, and emendation of the genus Thermus. Int J Syst Bacteriol 46:604–606
Pantazaki AA, Karagiorgas AA, Liakopoulou-Kyriakides M, Kyriakidis DA (1998) Hyperalkaline and thermostable phosphatase in Thermus thermophilus. Appl Biochem Biotechnol 75:249–259
Park T, Lee JH, Kim HK, Hoe HS, Kwon ST (1999) Nucleotide sequence of the gene for alkaline phosphatase of Thermus caldophilus GK24 and characteristics of the deduced primary structure of the enzyme. FEMS Microbiol Lett 180:133–139
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual (2nd edn). Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
Shine J, Dalgarno L (1975) Determination of cistron specificity in bacterial ribosomes. Nature 254:34–38
Yazynin S, Lange H, Mokros T, Deyev S, Lemke H (1999) A new phagemid vector for positive selection of recombinants based on a conditionally lethal barnase gene. FEBS Lett 452:351–354
Yeh MF, Trela JM (1976) Purification and characterization of a repressible alkaline phosphatase from Thermus aquaticus. J Biol Chem 251:3134–3139
Yuan YZ, Sheng XY, Lu HY, Tong S, Mao YM (1998) Thermostable alkaline phosphatase from Thermus sp. FD3041: cloning of the gene and expression in E. coli (in Japanese). Yi Chuan Xue Bao 25:375–380
Zappa S, Rolland JL, Flament D, Gueguen Y, Boudrant J, Dietrich J (2001) Characterization of a highly thermostable alkaline phosphatase from the euryarchaeon Pyrococcus abyssi. Appl Environ Microbiol 67:4504–4511
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. P. Georgiev
Rights and permissions
About this article
Cite this article
Yurchenko, J.V., Budilov, A.V., Deyev, S.M. et al. Cloning of an alkaline phosphatase gene from the moderately thermophilic bacterium Meiothermus ruber and characterization of the recombinant enzyme. Mol Gen Genomics 270, 87–93 (2003). https://doi.org/10.1007/s00438-003-0899-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00438-003-0899-y