Abstract
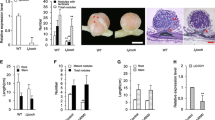
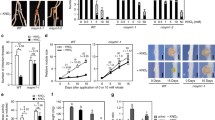
A Lotus japonicus mutant, Ljsym75, which forms ineffective symbiotic nodules and defines a new locus involved in the process of nitrogen fixation, was characterized in detail in order to identify the stage of developmental arrest of the nodules. No nitrogen-fixing activity was detectable in Ljsym75 nodules at any stage during plant development, and plant growth was markedly retarded. Ljsym75 plants formed twice as many nodules as the wild-type Gifu, and this phenotype was not influenced by the application of low concentrations of nitrate. Although the ineffective nodules formed on Ljsym75 were anatomically similar to effective Gifu nodules, Ljsym75 nodules senesced prematurely. Microscopic examination revealed that bacteria endocytosed into Ljsym75 nodules failed to differentiate into bacteroids. Moreover, the bacteria contained no nitrogenase proteins, whereas leghemoglobin was detected in the cytosol of the nodules. These results indicate that Ljsym75 is required for bacterial differentiation into nitrogen-fixing bacteroids in nodules, and thus the Ljsym75 gene was renamed sen1 (for stationary endosymbiont nodule). Linkage analysis using DNA markers showed that Sen1 is located on chromosome 4.








Similar content being viewed by others
References
Bénaben V, Duc G, Lefebvre V, Huguet T (1995) TE7, an inefficient symbiotic mutant of Medicago truncatula Gaertn. cv. Jemalong. Plant Physiol 107:53–62
Bensadoun A, Weinstein D (1976) Assay of proteins in the presence of interfering materials. Anal Biochem 70:241–250
Borisov AY, Morzhina EV, Kulikova OA, Tchetkova SA, Lebsky VK, Tikhonovich IA (1992) New symbiotic mutants of pea ( Pisum sativum L.) affecting either nodule initiation or symbiosome development. Symbiosis 14:297–313
Borisov AY, Rozov SM, Tsyganov VE, Morzhina EV, Lebsky VK, Tikhonovich IA (1997) Sequential functioning of Sym-13 and Sym-31, two genes affecting symbiosome development in root nodules of pea (Pisum sativum L.). Mol Gen Genet 254:592–598
Carroll BJ, McNeil DL, Gresshoff PM (1985) Isolation and properties of soybean [Glycine max (L.) Merr.] mutants that nodulate in the presence of high nitrate concentrations. Proc Natl Acad Sci USA 82:4162–4166
Endre G, Kereszt A, Kevei Z, Mihacea S, Kaló P, Kiss GB (2002) A receptor kinase gene regulating symbiotic nodule development. Nature 417:962–966
Gremaud MF, Harper JE (1989) Selection and initial characterization of partially nitrate tolerant nodulation mutants of soybean. Plant Physiol 89:169–173
Handberg K, Stougaard J (1992) Lotus japonicus, an autogamous, diploid legume species for classical and molecular genetics. Plant J 2:487–496
Häser A, Robinson DL, Duc G, Vance CP (1992) A mutation in Vicia faba results in ineffective nodules with impaired bacteroid differentiation and reduced synthesis of late nodulins. J Exp Bot 43:1397–1407
Hayashi M, et al (2001) Construction of a genetic linkage map of the model legume Lotus japonicus using an intraspecific F2 population. DNA Res 8:301–310
Imaizumi-Anraku H, Kawaguchi M, Koiwa H, Akao S, Syono K (1997) Two ineffective-nodulating mutants of Lotus japonicus—different phenotypes caused by the blockage of endocytotic bacterial release and nodule maturation. Plant Cell Physiol 38:871–881
Kawaguchi M (2000) Lotus japonicus 'Miyakojima' MG-20: an early-flowering accession suitable for indoor handling. J Plant Res 113:507–509
Kawaguchi M, Motomura T, Imaizumi-Anraku H, Akao S, Kawasaki S (2001) Providing the basis for genomics in Lotus japonicus: the accessions Miyakojima and Gifu are appropriate crossing partners for genetic analyses. Mol Genet Genomics 266:157–166
Kawaguchi M, Imaizumi-Anraku H, Koiwa H, Niwa S, Ikuta A, Syono K, Akao S (2002) Root, root hair, and symbiotic mutants of the model legume Lotus japonicus. Mol Plant-Microbe Interact 15:17–26
Kneen BE, LaRue TA, Hirsch AM, Smith CA, Weeden NF (1990) sym13—a gene conditioning ineffective nodulation in Pisum sativum. Plant Physiol 94:899–905
Kosambi DD (1944) The estimation of map distances from recombination values. Ann Eugen 12:172–175
Lerouge P, Roche P, Faucher C, Maillet F, Truchet G, Promé JC, Dénarié J (1990) Symbiotic host-specificity of Rhizobium meliloti is determined by a sulphated and acylated glucosamine oligosaccharide signal. Nature 344:781–784
Morzhina EV, Tsyganov VE, Borisov AY, Lebsky VK, Tikhonovich IA (2000) Four developmental stages identified by genetic dissection of pea (Pisum sativum L.) root nodule morphogenesis. Plant Sci 155:75–83
Nakamura Y, Kaneko T, Asamizu E, Kato T, Sato S, Tabata S (2002) Structural analysis of a Lotus japonicus genome. II. Sequence features and mapping of sixty-five TAC clones which cover a 6.5-Mb region of the genome. DNA Res 9:63–70
Novák K, Pesina K, Nebesárová J, Skrdleta V, Lisá L, Nasinec V (1995) Symbiotic tissue degradation pattern in the ineffective nodules of three nodulation mutants of pea (Pisum sativum L.). Ann Bot 76:303–313
Ohta T, Kato KH, Abe T, Takeuchi T (1993) Sperm morphology and distribution of intramembranous particles in the sperm heads of selected freshwater teleosts. Tissue Cell 25:725–735
Postma JG, Jager D, Jacobsen E, Feenstra WJ (1990) Studies on a non-fixing mutant of pea ( Pisum sativum L.). I. Phenotypical description and bacteroid activity. Plant Sci 68:151–161
Purdom D, Trese AT (1995) Morphological and molecular characteristics of host-conditioned ineffective root nodules in cowpea. Plant Physiol 109:239–244
Romanov VI, Gordon AJ, Minchin FR, Witty JF, Skot L, James CL, Borisov AY, Tikhonovich IA (1995) Anatomy, physiology and biochemistry of root nodules of Sprint-2 Fix-, a symbiotically defective mutant of pea (Pisum sativum L.). J Exp Bot 46:1809–1816
Romanov VI, Gordon AJ, Minchin FR, Witty JF, Skot L, James CL, Tikhonovich IA (1998) Physiological and biochemical characteristics of FN1, a 'fixation impaired' mutant of pea (Pisum sativum L.). J Exp Bot 49:1789–1796
Sagan M, Morandi D, Tarenghi E, Duc G (1995) Selection of nodulation and mycorrhizal mutants in the model plant Medicago truncatula (Gaertn.) after γ-ray mutagenesis. Plant Sci 111:63–71
Sato S, Kaneko T, Nakamura Y, Asamizu E, Kato T, Tabata S (2001) Structural analysis of a Lotus japonicus genome. I. Sequence features and mapping of fifty-six TAC clones which cover a 5.4 Mb region of the genome. DNA Res 8:311–318
Schauser L, Handberg K, Sandal N, Stiller J, Thykjaer T, Pajuelo E, Nielsen A, Stougaard J (1998) Symbiotic mutants deficient in nodule establishment identified after T-DNA transformation of Lotus japonicus. Mol Gen Genet 259:414–423
Schauser L, Roussis A, Stiller J, Stougaard J (1999) A plant regulator controlling development of symbiotic root nodules. Nature 402:191–195
Stracke S, Kistner C, Yoshida S, Mulder L, Sato S, Kaneko T, Tabata S, Sandal N, Stougaard J, Szczyglowski K, Parniske M (2002) A plant receptor-like kinase required for both bacterial and fungal symbiosis. Nature 417:959–962
Suganuma N (1999) Host-plant genes affecting nitrogen fixing activity in legume nodules. Curr Topics Plant Biol 1:145–149
Suganuma N, Tamaoki M, Kouchi H (1995) Expression of nodulin genes in plant-determined ineffective nodules of pea. Plant Mol Biol 28:1027–1038
Suganuma N, Sonoda N, Nakane C, Hayashi K, Hayashi T, Tamaoki M, Kouchi H (1998) Bacteroids isolated from ineffective nodules of Pisum sativum mutant E135 (sym13) lack nitrogenase activity but contain the two protein components of nitrogenase. Plant Cell Physiol 39:1093–1098
Szczyglowski K, Shaw RS, Wopereis J, Copeland S, Hamburger D, Kasiborski B, Dazzo FB, de Bruijn FJ (1998) Nodule organogenesis and symbiotic mutants of the model legume Lotus japonicus. Mol Plant-Microbe Interact 7:684–697
Vance CP, Johnson LEB (1983) Plant determined ineffective nodules in alfalfa (Medicago sativa): structural and biochemical comparisons. Can J Bot 61:93–106
Vance CP, Egli MA, Griffith SM, Miller SS (1988) Plant regulated aspects of nodulation and N2 fixation. Plant Cell Environ 11:413–427
Voroshilova VA, Boesten B, Tsyganov VE, Borisov AY, Tikhonovich IA, Priefer UB (2001) Effect of mutations in Pisum sativum L. genes blocking different stages of nodule development on the expression of late symbiotic genes in Rhizobium leguminosarum bv. viciae. Mol Plant-Microbe Interact 14:471–476
Acknowledgements
The authors would like to thank Dr. F.C. Guinel (Wilfrid Laurier University, Laval, Canada) for critical reading of the manuscript and Dr. T. Bisseling (Agricultural University, Wageningen, Netherlands) for providing antisera against nitrogenase components I and II from pea bacteroids. They would also like to acknowledge Dr. S. Sato (Kazusa DNA Research Institute) for providing information about DNA markers, Dr. H. Kouchi (National Institute of Agrobiological Resources) and Dr. H. Kitano (Nagoya University) for technical suggestions. This work was supported by special coordination funds for promoting science and technology from the Japanese Ministry of Education, Culture, Sports, Science, and Technology.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Kondorosi
Rights and permissions
About this article
Cite this article
Suganuma, N., Nakamura, Y., Yamamoto, M. et al. The Lotus japonicus Sen1 gene controls rhizobial differentiation into nitrogen-fixing bacteroids in nodules. Mol Gen Genomics 269, 312–320 (2003). https://doi.org/10.1007/s00438-003-0840-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00438-003-0840-4