Abstract
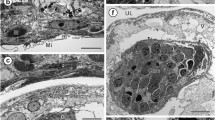
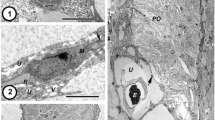
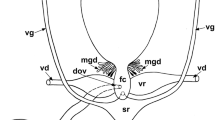
Ultrastructural studies of the monogenean uterus are few in number and no non-polystomatid polyopisthocotyleans have been investigated. The uterus of Chimaericola leptogaster, a basal polyopisthocotylean monogenean, has several unusual features, including six reflexed loops comprising four ascending and three descending, longitudinally oriented, linear sections. At the ultrastructural level, three readily distinguishable uterine regions and other distinctive characteristics are apparent. One novel feature occurs in the proximal uterus, where the lining forms a so-called ‘single-layered multi-rowed cellular epithelium’, which includes two types of cells, tall (ca. 14–19 μm in height) and short (ca. 6–9 μm in height) cells, both lying on the basement membrane. Although known from other bilaterian groups, this is the first record of this type of epithelium in the Neodermata. The lining of the middle uterine region comprises a single regular layer of columnar glandular epithelial cells, which produce numerous rounded, electron-dense granules associated with Golgi complexes. The presence of the uterine glands in the middle region of the uterus is an unusual feature for a monogenean, having previously been described only for basal orders of the Cestoda, i.e. the Gyrocotylidea, Caryophyllidea and Spathebothriidea. Seen in cross-section, the epithelium of the distal uterus contains three areas of tall single-layered columnar epithelium (ca. 30 μm deep) interspersed by three areas of flattened epithelium (ca. 0.2–0.9 μm deep). Such a pattern is quite different from those reported for other monogeneans and, indeed, other neodermatan groups. The investigation has shown that the outer layer of the fully formed eggshell is assembled from epithelial secretions in the middle uterine lumen, but is modified in terms of its shape in the distal uterus. Possible phylogenetic implications arising from the unusual features described are discussed in relation to other neodermatan groups and recent molecular phylogenies of the Bilateria.








Similar content being viewed by others
References
Aguinaldo AM, Turbeville JM, Linford LS, Rivera MC, Garey JR, Raff RA, Lake JA (1997) Evidence for a clade of nematodes, arthropods and other moulting animals. Nature 387:489–493. do:10.1038/387489a0
Baguñà J, Riutort M (2004) The dawn of bilaterian animals: the case of acoelomorph flatworms. BioEssays 26:1046–1057. doi:10.1002/bies.20113
Beverley-Burton M, Chisholm LF, Last P (1991) Two new species of Chimaericola Brinkmann (Monogenea: Chimaericolidae) from Hydrolagus spp. (Chimaeriformes: Chimaeridae) in the Pacific. Syst Parasitol 18:59–66. doi:10.1007/bf00012224
Boeger WA, Kritsky DC (1993) Phylogeny and a revised classification of the Monogenoidea Bychowsky, 1937 (Platyhelminthes). Syst Parasitol 26:1–32. doi:10.1007/BF00009644
Boeger WA, Kritsky DC (1997) Coevolution of the Monogenoidea (Platyhelminthes) based on a revised hypothesis of parasite phylogeny. Int J Parasitol 27:1495–1511. doi:10.1016/S0020-7519(97)00140-9
Boeger WA, Kritsky DC (2001) Phylogenetic relationships of the Monogenoidea. In: Littlewood DTJ, Bray RA (eds) Interrelationships of the Platyhelminthes. Taylor & Francis, London, pp 92–102
Brinkmann A Jr (1942) On “Öctobothrium” leptogaster F.S. Leuckart. Göteborgs Kungl. Vetenskaps och Vitterhets Samhälles Handlingar, Ser B 2:3–29 Sjätte Följden, Göteborg, Flanders Boktryckeri Akliebolag
Burton PR (1963) A histochemical study of vitelline cells, egg capsules, and Mehlis’ gland in the frog lung-fluke Haematoloechus medioplexus. J Exper Zool 154:247–257. doi:10.1002/jez.1401540212
Bychowsky BE (1957) Monogenetic trematodes, their systematics and phylogeny. Izdatel'stvo Academiya Nauk SSSR, Moscow (In Russian)
Cable J, Tinsley RC (1991) Intra-uterine larval development of the polystomatid monogeneans, Pseudodiplorchis americanus and Neodiplorchis scaphiopodis. Parasitology 103:253–266. doi:10.1017/s0031182000059539
Cable J, Harris PD, Tinsley RC (1996) Ultrastructural adaptations for viviparity in the female reproductive system of gyrodactylid monogeneans. Tissue Cell 28:515–526. doi:10.1016/s0040-8166(96)80054-1
Cable J, Tocque K, Tinsley RC (1997) Histological analysis of the egg capsule of the ovoviviparous polystomatid monogenean Pseudodiplorchis americanus. Int J Parasitol 27:1075–1080. doi:10.1016/s0020-7519(97)00068-4
Caira JN, Littlewood DTJ (2013) Worms, Platyhelminthes. In: Levin SA (ed.) Encyclopedia of biodiversity,. Academic Press, Waltham, MA, USA Vol. 7 pp 437–469
Colhoun IM, Fairweather I, Brennan GP (1998) Observations on the mechanism of eggshell formation in the liver fluke Fasciola hepatica. Parasitology 116:555–567. doi:10.1017/s0031182098002662
Davydov VG, Poddubnaya LG (1988) Functional morphology of the frontal and uterine glands in cestodes of the order Caryophyllidea. Parazitologiya 22:449–457 (In Russian)
Davydov VG, Poddubnaya LG, Kuperman BI (1997) An ultrastructure of some systems of Dictyocotyle olrikii (Cestoda: Cyathocephalata) in relation to peculiarities of its life cycle. Parazitologiya 31:132–141 (In Russian)
Dunn CW, Hejnol A, Matus DQ, Pang K, Browne WE, Smith SA, Seaver E, Rouse GW, Obst M, Edgecombe GD et al (2008) Broad phylogenomic sampling improves resolution of the animal tree of life. Nature 452:745–749. doi:10.1038/nature06614
Egger B, Lapraz F, Tomiczek B, Müller S, Dessimoz C, Girstmair J, Škunca N, Rawlinson KA, Cameron CB, Beli E, Todaro MA, Gammoudi M, Noreña C,Telford1 MJ (2015) A transcriptomic-phylogenomic analysis of the evolutionary relationships of flatworms. Curr Biol 25: 1347–1353. doi:10.1016/j.cub.2015.03.034
Ehlers U (1985) Das Phylogenetische System der Platyhelminthes. Gustav Fischer, Stuttgart
El-Naggar MM, Khidr AA, Kearn GC (1990) Ultrastructural observations on the oviduct, Mehlis' glands and ootype of the monogenean Cichlidogyrus halli typicus (Price & Kirk, 1967) Paperna, 1979. Int J Parasitol 20:203–209. doi:10.1016/0020-7519(90)90102-s
Gremigni V (1983) Platyhelminthes–Turbellaria. In: Adiyodi KG, Adiyodi RG (eds) Reproductive biology of invertebrates, Oogenesis, oviposition and oosorption, vol 1. John Wiley & Sons Ltd, Chichester, pp 67–107
Guraya SS, Parshad VR (1988) Platyhelminthes. In: Reproductive biology of invertebrates. Accessory sex glands. John Wiley & Sons, Chichester, Vol. 3 pp 1–49
Halanych KM, Bacheller JD, Aguinaldo AM, Liva SM, Hillis DM, Lake JA (1995) Evidence from 18S ribosomal DNA that the lophophorates are protostome animals. Science 267:1641–1643. doi:10.1126/science.7886451
Ham AW, Cormack DH (1979) Histology, Eighth edn. J.B. Lippincott Company, Philadelphia and Toronto
Hathaway RP, Herlevich JC (1976) A histochemical study of egg shell formation in the monogenetic trematode Octomacrum lanceatum Mueller, 1934. Proc Helminthol Soc Wash 43:203–206
Hejnol A, Obst M, Stamatakis A, Ott M, Rouse GW, Edgecombe GD et al (2009) Assessing the root of bilaterian animals with scalable phylogenomic methods. Proc Roy Soc B, Biol Sci 276:4261–4270. doi:10.1098/rspb.2009.0896
Inoue JG, Miya M, Lam K, Tay BH, Danks JA, Bell J, Walker TI, Venkatesh B (2010) Evolutionary origin and phylogeny of the modern holocephalans (Chondrichthyes: Chimaeriformes): a mitogenomic perspective. Mol Biol Evol 27:2576–2586. doi:10.1093/molbev/msq147
Irwin SWB, Threadgold LT (1972) Electron-microscope studies on Fasciola hepatica. X Egg formation Exp Parasitol 31:321–331. doi:10.1016/0014-4894(72)90093-8
Jones MK, Ernst I, Whittington ID (1998) The uterine epithelium of Gyrodactylus kobayashii (Monogenea: Gyrodactylidae): ultrastructure of basal matrices, cytoplasmic membranes and the birth plug, and comparison with other reproductive epithelia. Int J Parasitol 28:1805–1815. doi:10.1016/s0020-7519(98)00120-9
Jovelin R, Justine J-L (2001) Phylogenetic relationships within the polyopisthocotylean monogeneans (Platyhelminthes) inferred from partial 28S rDNA sequences. Int J Parasitol 31:393–401. doi:10.1016/S0020-7519(01)00114-X
Justine J-L, Mattei X (1986) Ultrastructural observations on fertilization in Dionchus remorae (Platyhelminthes, Monogenea, Dionchidae). Acta Zool 67:97–101. doi:10.1111/j.1463-6395.1986.tb00853.x
Kearn GC (1986) The eggs of monogeneans. Adv Parasitol 25:175–273. doi:10.1016/S0065-308X(08)60344-9
Ktari MH (1971) Recherches sur la reproduction et le développement de quelques monogènes (Polyopisthocotylea) parasites de poissons marins. Université des Sciences et Techniques du Languedoc, Thesis
Laumer CE, Giribet G (2014) Inclusive taxon sampling suggests a single, stepwise origin of ectolecithality in Platyhelminthes. Biol J Linn Soc 111:570–588. doi:10.1111/bij.12236
Laumer CE, Hejnol A, Giribet G (2015). Nuclear genomic signals of the‘microturbellarian’ roots of platyhelminth evolutionary innovation. eLife 4,e05503. doi:10.7554/elifesciences.org05503
Littlewood DTJ, Waeschenbach A (2015) Evolution: a turn up for the worms. Curr Biol 25:R448–R469. doi:10.1016/j.cub.2015.04.012
Littlewood DTJ, Rohde K, Clough KA (1999) The interrelationships of all major groups of Platyhelminthes: phylogenetic evidence from morphology and molecules. Biol J Linn Soc 66:75–114. doi:10.1111/j.1095-8312.1999.tb01918.x
Mollaret I, Jamieson BGM, Justine J-L (2000) Phylogeny of the Monopisthocotylea and Polyopisthocotylea (Platyhelminthes) inferred from 28S rDNA sequences. Int J Parasitol 30:171–185. doi:10.1016/S0020-7519(01)00114-X
Poddubnaya LG, Hemmingsen W (2014) Cytoarchitecture of the vitellaria of two monogenean species, parasites of the holocephalan fish, Chimaera monstrosa L, with analysis of vitelline structure in the Neodermata. Parazitologiya 48:257–269 (In Russian)
Poddubnaya LG, Mackiewicz JS, Kuperman BI (2003) Ultrastructure of Archigetes sieboldi (Cestoda: Caryophyllidea): relationship between progenesis, development and evolution. Folia Parasitol 50:275–292. doi:10.14411/fp.2003.047
Poddubnaya LG, Mackiewicz JS, Bruňanská M, Scholz T (2005a) Fine structure of the female reproductive ducts of Cyathocephalus truncatus (Cestoda: Spathebothriidea), from salmonid fish. Folia Parasitol 52:323–338. doi:10.14411/fp.2005.045
Poddubnaya LG, Mackiewicz JS, Šwiderski Z, Bruňanská M, Scholz T (2005b) Fine structure of egg-forming complex ducts, eggshell formation and supporting neuronal plexus in progenetic Diplocotyle olrikii (Cestoda, Spathebothriidea). Acta Parasitol 50:292–304
Poddubnaya LG, Kuchta R, Levron C, Gibson DI, Scholz T (2009) The unique ultrastructure of the uterus of the Gyrocotylidea Poche, 1926 (Cestoda) and its phylogenetic implications. Syst Parasitol 74:81–93. doi:10.1007/s11230-009-9195-5
Poddubnaya LG, Hemmingsen W, Gibson DI (2013) Ultrastructural characteristics of the vaginae of the basal monogenean Chimaericola leptogaster (Leuckart, 1830). Parasitol Res 112:4053–4064. doi:10.1007/s00436-013-3596-8
Poddubnaya LG, Hemmingsen W, Gibson DI (2014) Clamp ultrastructure of the basal monogenean Chimaericola leptogaster (Leuckart, 1830) (Polyopisthocotylea: Chimaericolidae). Parasitol Res 113:4023–4032. doi:10.1007/s00436-014-4070-y
Poddubnaya LG, Hemmingsen W, Reed C, Gibson DI (2015) Ultrastructural characteristics of the caeca of basal polyopisthocotylean monogeneans of the families Chimaericolidae and Hexabothriidae parasitic on cartilaginous fishes. Parasitol Res 114:2599–2610. doi:10.1007/s00436-015-4464-5
Rieger RM (1974) A new group of Turbellaria–Typhloplanoida with a proboscis and its relationship to Kalyptorhynchia. In: Riser NW, Morse MP (eds) Biology of the Turbellaria. McGraw-Hill, New York, pp 17–62
Shinn GL, Christensen AM (1985) Kronborgia pugettensis sp.nov. (Neorhabdocoela: Fecampiidae), an endoparasitic turbellarian infesting the shrimp Heptacarpus kincaidi (Rathbun), with notes on its life-history. Parasitology 91:431–447. doi:10.1017/s0031182000062685
Schmidt J (1996) Complex carbohydrates in shell precursor globules of the vitellarium and at the eggshell of Hymenolepis microstoma (Cestoda). Parasitol Res 82:157–164. doi:10.1007/s004360050087
Schmidt J (1998) Glycan vesicle formation in vitellocytes and hatching vacuoles in eggs of Echinostoma caproni and Fasciola hepatica (Digenea). Tissue Cell 30:416–426. doi:10.1016/s0040-8166(98)80056-6
Smyth JD, Halton DW (1983) The physiology of trematodes. Cambridge University Press, Cambridge
Stranock SD, Halton DW (1975) Ultrastructural observations on Mehlis’ gland in the monogeneans, Diplozoon paradoxum and Calicotyle kroyeri. Int J Parasitol 5:541–550. doi:10.1016/0020-7519(75)90047-8
Tappenden T, Kearn GC, Evans-Gowing R (1993) Fertilization and the functional anatomy of the germarium in the monogenean Entobdella soleae. Int J Parasitol 23:901–911. doi:10.1016/0020-7519(93)90056-5
Wells KE, Cordingley JS (1991) Schistosoma mansoni: eggshell formation is regulated by pH and calcium. Exp Parasitol 73:295–231. doi:10.1016/0014-4894(91)90101-2
Zavarzin AA (1985) [Foundations of comparative histology.] Leningrad, Leningrad state university. Publishing house to Leningrad State University, pp. 400. (In Russian)
Acknowledgements
The authors would like to thank the staff of the RV ‘Johan Ruud’, belonging to Tromsø University (Norway), for their invaluable help with the fishing. We are also grateful to the staff of the Centre of Electron Microscopy, I.D. Papanin Institute for Biology of Inland Waters (Borok, Russia) for technical assistance. The present study was supported by the Russian Foundation for Fundamental research (Project no.15-04-02890-a to LGP).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Poddubnaya, L.G., Hemmingsen, W. & Gibson, D.I. The unique uterine structure of the basal monogenean Chimaericola leptogaster (Monogenea: Polyopisthocotylea), an ectoparasite of the relictual holocephalan fish Chimaera monstrosa . Parasitol Res 116, 2695–2705 (2017). https://doi.org/10.1007/s00436-017-5578-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-017-5578-8