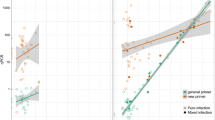
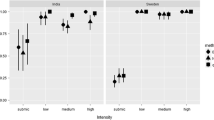
Abstract
Although wildlife rehabilitation and translocations are important tools in wildlife conservation in New Zealand, disease screening of birds has not been standardized. Additionally, the results of the screening programmes are often difficult to interpret due to missing disease data in resident or translocating avian populations. Molecular methods have become the most widespread method for diagnosing avian malaria (Plasmodium spp.) infections. However, these methods can be time-consuming, expensive and are less specific in diagnosing mixed infections. Thus, this study developed a new real-time PCR (qPCR) method that was able to detect and specifically identify infections of the three most common lineages of avian malaria in New Zealand (Plasmodium (Novyella) sp. SYAT05, Plasmodium elongatum GRW6 and Plasmodium spp. LINN1) as well as a less common, pathogenic Plasmodium relictum GRW4 lineage. The assay was also able to discern combinations of these parasites in the same sample and had a detection limit of five parasites per microlitre. Due to concerns relating to the presence of the potentially highly pathogenic P. relictum GRW4 lineage in avian populations, an additional confirmatory high resolution (HRM) qPCR was developed to distinguish between commonly identified P. elongatum GRW6 from P. relictum GRW4. The new qPCR assays were tested using tissue samples containing Plasmodium schizonts from three naturally infected dead birds resulting in the identified infection of P. elongatum GRW6. Thus, these rapid qPCR assays have shown to be cost-effective and rapid screening tools for the detection of Plasmodium infection in New Zealand native birds.





Similar content being viewed by others
Notes
Kate McInnes, Department of Conservation, Wellington, New Zealand. kmcinnes@doc.govt.nz
References
Alley MR, Fairley RA, Martin DG, Howe L, Atkinson T (2008) An outbreak of avian malaria in captive yellowheads/mohua (Mohoua ochrocephala). New Zeal Vet J 56:247–251
Alley MR, Hale KA, Cash W, Ha HJ, Howe L (2010) Concurrent avian malaria and avipox virus infection in translocated South Island saddlebacks (Philesturnus carunculatus carunculatus). New Zeal Vet J 58:218–223
Arriero E, Moller AP (2008) Host ecology and life-history traits associated with blood parasite species richness in birds. J Evol Bio 21:1504–1513
Atkinson CT, van Riper C (1991) Pathogenicity and epizootiology of avian haematozoa: Plasmodium, Leucocytozoon, and Haemoproteus. In: Loye JE, Zuk M (eds) Bird-parasite interactions: ecology, evolution, and behavior. Oxford University Press, Oxford, pp 19–48
Atkinson CT, Dusek RJ, Woods KL, Iko WM (2000) Pathogenicity of avian malaria in experimentally-infected Hawaii Amakihi. J Wildlife Dis 36:197–204
Banda ME, Howe L, Gartrell BD, Mcinnes K, Hunter S, French NP (2013) A cluster of avian malaria cases in a kiwi management programme. New Zeal Vet J 61:121–126
Baron HR, Howe L, Varsani A, Doneley RJT (2014) Disease screening of three breeding populations of adult exhibition budgerigars (Melopsittacus undulatus) in New Zealand reveals a high prevalence of a novel polyomavirus and avian malaria infection. Avian Dis 58:111–117
Bell JA, Weckstein JD, Fecchio A, Tkach VV (2015) A new real-time PCR protocol for detection of avian haemosporidians. Parasite Vector. doi:10.1186/s13071-015-0993-0
Bennett GF, Peirce MA, Ashford RW (1993) Avian hematozoa—mortality and pathogenicity. J Nat Hist 27:993–1001
Bensch S, Hellgren O, Pérez-Tris J (2009) MalAvi: a public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Mol Ecol Resour 9:1353–1358. doi:10.1111/j.1755-0998.2009.02692.x
Bentz S, Rigaud T, Barroca M, Martin-Laurent F, Bru D, Moreau J, Faivre B (2006) Sensitive measure of prevalence and parasitaemia of haemosporidia from European blackbird (Turdus merula) populations: value of PCR-RFLP and quantitative PCR. Parasitology 133:685–692. doi:10.1017/s0031182006001090
Castro IC, Howe L, Tompkins DM, Barraclough RK, Slaney D (2011) Presence and seasonal prevalence of Plasmodium spp. in a rare endemic New Zealand passerine. (Tieke or saddleback, Philesturnus carunculatus) J Wildlife Dis 47(4):860–867
Clarke JR, Kerry KR (1993) Diseases and parasites of penguins. Kor J Polar Res 4:79–96
Craig J, Anderson S, Clout M, Creese B, Mitchell N, Ogden J, Roberts M, Ussher G (2000) Conservation issues in New Zealand. Annu Rev Ecol Syst 31:61–78
Cunningham AA, Langton TES, Bennett PM, Lewin JF, Drury SEN, Gough RE, Macgregor SK (1996) Pathological and microbiological findings from incidents of unusual mortality of the common frog (Rana temporaria). Philos T R Soc Lon B 351:1539–1557. doi:10.1098/rstb.1996.0140
Dimitrov D, Palinauskas V, Iezhova TA, Ilgūnas M, Bukauskaitė D, Zehtindjiev P, Ilieva M, Shapoval AP, Bolshakov CV, Markovets MY, Bensch S, Valkiūnas G (2015) Plasmodium spp.: an experimental study on vertebrate host susceptibility to avian malaria. Exp Parasitol 148:1–16
Dinhopl N, Nedorost N, Mostegl MM, Weissenbacher-Lang C, Weissenböck H (2015) In situ hybridization and sequence analysis reveal an association of Plasmodium spp. with mortalities in wild passerine birds in Austria. Parasitol Res 114:1455–1462. doi:10.1007/s00436-015-4328-z
Ewen JG, Armstrong DP, Empson R, Jack S, Makan T, Mcinnes K, Parker KA, Richardson K, Alley M (2012) Parasite management in translocations: lessons from a threatened New Zealand bird. Oryx 46:446–456. doi:10.1017/s0030605311001281
Fallon SM, Ricklefs RE, Swanson BL, Bermingham E (2003) Detecting avian malaria: an improved polymerase chain reaction diagnostic. J Parasitol 89:1044–1047
Farrugia C, Cabaret O, Botterel F, Bories C, Foulet F, Costa JM, Bretagne S (2011) Cytochrome b gene quantitative PCR for diagnosing Plasmodium falciparum infection in travelers. J Clin Microbiol 49:2191–2195
Friedl TWP, Groscurth E (2012) A real-time PCR protocol for simple and fast quantification of blood parasite infections in evolutionary and ecological studies and some data on intensities of blood parasite infections in a subtropical weaverbird. J Ornithol 153:239–247. doi:10.1007/s10336-011-0735-9
Hellgren O, Waldenström J, Bensch S (2004) A new PCR assay for simultaneous studies of Leucocytozoon, Plasmodium, and Haemoproteus from avian blood. J Parasitol 90:797–802
Hellgren O, Atkinson CT, Bensch S, Albayrak T, Dimitrov D, Ewen JG, Kim KS, Lima MR, Martin L, Palinauskas V (2015) Global phylogeography of the avian malaria pathogen Plasmodium relictum based on MSP1 allelic diversity. Ecography 38(8):842–850
Howe L, Castro IC, Schoener ER, Hunter S, Barraclough RK, Alley MR (2012) Malaria parasites (Plasmodium spp.) infecting introduced, native and endemic New Zealand birds. Parasitol Res 110:913–923. doi:10.1007/s00436-011-2577-z
Jarvi SI, Schultz JJ, Atkinson CT (2002) PCR diagnostics underestimate the prevalence of avian malaria (Plasmodium relictum) in experimentally-infected passerines. J Parasitol 88:153–158
Jarvi SI, Farias ME, Lapointe DA, Belcaid M, Atkinson CT (2013) Next-generation sequencing reveals cryptic mtDNA diversity of Plasmodium relictum in the Hawaiian Islands. Parasitology 140:1741–1750. doi:10.1017/s0031182013000905
Jones HI, Shellam GR (1999) Blood parasites in penguins, and their potential impact on conservation. Mar Ornithol 27:181–184
Knowles SCL, Wood MJ, Alves R, Wilkin TA, Bensch S, Sheldon BC (2011) Molecular epidemiology of malaria prevalence and parasitaemia in a wild bird population. Molec Ecol 20:1062–1076. doi:10.1111/j.1365-294X.2010.04909.x
Learngaramkul P, Petmitr S, Krungkrai SR, Prapunwattana P, Krungkrai J (1999) Molecular characterization of mitochondria in asexual and sexual blood stages of Plasmodium falciparum. Mol Cell Biol Res Commun 2:15–20
Madigan M, Martinko J, Bender KS, Buckley DH, Stahl DA (2015) Brock biology of microorganisms. Pearson, Boston
Marzal A, Bensch S, Reviriego M, Balbontin J, De Lope F (2008) Effects of malaria double infection in birds: one plus one is not two. J Evolution Biol 21:979–987. doi:10.1111/j.1420-9101.2008.01545.x
Mathews F, Moro D, Strachan R, Gelling M, Buller N (2006) Health surveillance in wildlife reintroductions. Biol Conserv 131:338–347. doi:10.1016/j.biocon.2006.04.011
Musset L, Pradines B, Parzy D, Durand R, Bigot P, Le Bras J (2006) Apparent absence of atovaquone/proguanil resistance in 477 Plasmodium falciparum isolates from untreated French travellers. J Anitmicrob Chemoth 57:110–115
Njabo KY, Cornel AJ, Bonneaud C, Toffelmier E, Sehgal RNM, Valkiūnas G, Russell AF, Smith TB (2011) Nonspecific patterns of vector, host and avian malaria parasite associations in a central African rainforest. Mol Ecol 20:1049–1061. doi:10.1111/j.1365-294X.2010.04904.x
Palinauskas V, Valkiūnas G, Bolshakov CV, Bensch S (2011) Plasmodium relictum (lineage SGS1) and Plasmodium ashfordi (lineage GRW2): the effects of the co-infection on experimentally infected passerine birds. Exp Parasitol 127:527–533. doi:10.1016/j.exppara.2010.10.007
Palinauskas V, Žiegyte R, Ilgūnas M, Iezhova TA, Bernotienė R, Bolshakov C, Valkiūnas G (2015) Description of the first cryptic avian malaria parasite, Plasmodium homocircumflexum n. sp., with experimental data on its virulence and development in avian hosts and mosquitoes. Int J Parasitol 45:51–62. doi:10.1016/j.ijpara.2014.08.012
Palinauskas V, Žiegytė R, Iezhova TA, Ilgūnas M, Bernotienė R, Valkiūnas G (2016) Description, molecular characterisation, diagnostics and life cycle of Plasmodium elongatum (lineage pERIRUB01), the virulent avian malaria parasite. Int J Parasitol 46(11):697–707
Parsons NJ, Underhill LG (2005) Oiled and injured African penguins Spheniscus demersus and other seabirds admitted for rehabilitation in the Western Cape, South Africa, 2001 and 2002. Afr J Mar Sci 27:289–296
Richard FA, Sehgal RNM, Jones HI, Smith TB (2002) A comparative analysis of PCR-based detection methods for avian malaria. J Parasitol 88:819–822
Schoener ER, Banda M, Howe L, Castro IC, Alley MR (2014) Avian malaria in New Zealand. New Zeal Vet J 62:189–198. doi:10.1080/00480169.2013.871195
Sturrock HJW, Tompkins DM (2008) Avian malaria parasites (Plasmodium spp.) in Dunedin and on the Otago Peninsula, southern New Zealand. New Zeal J Ecol 32(1):98–102
Thorne JM (2007) An experimental approach to the translocation of the north island saddleback (Philesturnus carunculatus rufusater) to Bushy Park Reserve, Wanganui. Massey University, Masters Dissertation
Tompkins DM, Gleeson DM (2006) Relationship between avian malaria distribution and an exotic invasive mosquito in New Zealand. J Royal Soc New Zeal 36:51–62
Tran TM, Aghili A, Li SP, Ongoiba A, Kayentao K, Doumbo S, Traore B, Crompton PD (2014) A nested real-time PCR assay for the quantification of Plasmodium falciparum DNA extracted from dried blood spots. Malar J 13:393. doi:10.1186/1475-2875-13-393
Valkiūnas G (2005) Avian malaria parasites and other haemosporidia. CRC, Florida
Valkiūnas G, Bensch S, Iezhova TA, Krizanauskiené A, Hellgren O, Bolshakov CV (2006) Nested cytochrome b polymerase chain reaction diagnostics underestimate mixed infections of avian blood haemosporidian parasites: microscopy is still essential. J Parasitol 92:418–422
Valkiūnas G, Palinauskas V, Ilgūnas M, Bukauskaitė D, Dimitrov D, Bernotienė R, Zehtindjiev P, Ilieva M, Iezhova TA (2014) Molecular characterization of five widespread avian haemosporidian parasites (Haemosporida), with perspectives on the PCR-based detection of haemosporidians in wildlife. Parasitol Res 113:2251–2263. doi:10.1007/s00436-014-3880-2
Vanstreels RE, da Silva-Filho RP, Kolesnikovas CK, Bhering RC, Ruoppolo V, Epiphanio S, Amaku M, Ferreira Junior FC, Braga EM, Catao-Dias JL (2015) Epidemiology and pathology of avian malaria in penguins undergoing rehabilitation in Brazil. Vet Res 46:30 doi:3010.1186/s13567–015–0160-9
Waldenström J, Bensch S, Hasselquist D, Ostman O (2004) A new nested polymerase chain reaction method very efficient in detecting Plasmodium and Haemoproteus infections from avian blood. J Parasitol 90(1):191–194
Wampfler R, Mwingira F, Javati S, Robinson L, Betuela I, Siba P, Beck HP, Mueller I, Felger I (2013) Strategies for detection of Plasmodium species gametocytes. Plos One, doi: e76316 10.1371/journal.Pone.0076316
Acknowledgements
We thank Dr. Isabel Castro and Dr. Dan Tompkins for their valuable suggestions to this manuscript. This project received funding from the Morris Animal Foundation study grant ID number D13ZO-811: Do Translocations for Species Restoration Cause Pathogen Pollution? Further funding was received from Julie Alley Bursary, Marion Cunningham Memorial Fund, Forest and Bird J S Watson Trust, Massey University (IAE) and the New Zealand Veterinary Association Wildlife Society.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All birds were caught at Bushy Park/Wanganui in February 2013 during a study examining avian malaria under the Department of Conservation permit TW-32756-FAU and the approval by the Animal Ethics Committee at Massey University, MUAEC Protocol 11/59. Birds were banded under institutional banding permit No. 2012/009.
Rights and permissions
About this article
Cite this article
Schoener, E.R., Hunter, S. & Howe, L. Development of a rapid HRM qPCR for the diagnosis of the four most prevalent Plasmodium lineages in New Zealand. Parasitol Res 116, 1831–1841 (2017). https://doi.org/10.1007/s00436-017-5452-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-017-5452-8