Abstract
Fasciolosis, caused by the liver fluke Fasciola hepatica, is a major parasitic disease of livestock that causes significant economic losses worldwide. Although drugs are effective against liver flukes, they do not prevent reinfection, and continuous treatment is costly. Moreover, resistant fluke strains are emerging. In this context, vaccination is a good alternative since it provides a cost-effective long-term prevention strategy to control fasciolosis. In this paper, we evaluate the Fhmuc peptide as a potential vaccine against fasciolosis. This peptide derives from a mucin-like protein highly expressed in the infective stage of Fasciola hepatica. Mucin-like molecules expressed by parasites can contribute to several infection processes by protecting the parasite from host proteases and recognition by the immune system. We show that the Fhmuc peptide induces Th1-like immune responses specific for F. hepatica excretion-secretion products (FhESP) with a high production of IFNγ. We also investigated whether this peptide could protect animals from infection, and present preliminary data indicating that animals treated with Fhmuc exhibited reduced liver damage compared to non-immunised animals and that this protection was associated with a recruitment of B and T lymphocytes in the peritoneum, as well as eosinophils and mature dendritic cells. These results suggest that the mucin-like peptide Fhmuc could constitute a potential vaccine candidate against fasciolosis and pave the way towards the development of vaccines against parasites.
Similar content being viewed by others
Introduction
Fasciolosis is a major parasitic disease of livestock that causes significant economic losses worldwide (Rojo-Vázquez et al. 2012). Currently, fasciolosis caused by the liver fluke Fasciola hepatica is considered an emerging zoonosis, with an increasing number of human infections globally (Rojo-Vázquez et al. 2012). During infection, this pathogen induces potent polarised Th2 and regulatory T cell immune responses, downregulating the production of Th1 cytokines (Donnelly et al. 2008; Flynn et al. 2007; O’Neill et al. 2000; Walsh et al. 2009). Thus, the parasite is able to modulate the host immune response by increasing the levels of IL-4, IL-5, IL-10, and TGFβ (Flynn and Mulcahy 2008; Walsh et al. 2009) and inhibiting the production of IFNγ or IL-17 (O’Neill et al. 2000; Walsh et al. 2009). This strategy allows the parasite to establish chronic infections and prolongs its survival in the host. Also, the immune regulation caused by liver fluke infection has been shown to increase susceptibility to other infectious diseases, such as bovine tuberculosis, thus affecting the efficacy of control programs (Claridge et al. 2012).
Although drugs such as triclabendazole (the current drug of choice) are effective against flukes, they do not prevent reinfection, and continuous treatment is costly (Fairweather 2009, 2011; Keiser et al. 2005). Furthermore, resistance to triclabendazole has been reported in livestock farms across Europe and Australia (Fairweather 2009; Keiser et al. 2005). Thus, new alternatives to chemotherapy are needed. Among them, vaccines would provide a cost-effective long-term prevention strategy to control fasciolosis (Fairweather 2011; Khan et al. 2013; Piedrafita et al. 2010).
To date, the majority of vaccination studies with either purified native or recombinant proteins from F. hepatica have been carried out using proteases, haemoglobin, glutathione S-transferase, or fatty acid binding proteins as immunogens (Hillyer 2005; McManus and Dalton 2006; Toet et al. 2014). Despite the existence of these vaccine candidates, there is currently no commercial vaccine available for fasciolosis. Most experimental vaccine trials with liver fluke vaccine candidates have been performed using recombinant antigens derived from adult parasites (Molina-Hernandez et al. 2015; Toet et al. 2014). Nevertheless, the use of antigens specific for juvenile stages would be of interest, since migrating parasites at this stage cause the most severe damage and pathology in liver fluke infections.
Mucin-like molecules expressed by parasites can contribute to several infection processes, including attachment to and invasion of host cells, by protecting the parasite from host proteases and recognition by the immune system (Theodoropoulos et al. 2001). For instance, the major components produced by the infective larvae of the nematode Toxocara canis include a family of mucin-like proteins that participate in immune evasion (Loukas et al. 2000; Maizels 2013; Maizels et al. 2000). In addition, the surface of the protozoan parasite Trypanosoma cruzi is covered with mucins, which contribute to parasite protection and to the establishment of persistent infections (Buscaglia et al. 2006). Finally, a highly polymorphic mucin family protein expressed by Schistosoma mansoni miracidia is important in assuring compatibility in the invertebrate host (Perrin et al. 2013; Roger et al. 2008).
During the characterisation of the transcriptome of F. hepatica newly excysted juveniles (NEJs), a cDNA clone coding for a mucin-like protein was identified (Cancela et al. 2010). The putative protein, characterised by repeated Ser and Thr residues predicted to be O-glycosylated, is the most abundant gene transcript in juvenile expressed sequence tags (Cancela et al. 2010, 2015). Considering that this transcript is highly expressed in the NEJ infective stage of the parasite, it would be interesting to test its immunoprophylactic potential, since a vaccine targeting juveniles could reduce invasion of the liver parenchyma and minimise liver pathology.
In this work, we investigated whether a mucin-like peptide, Fhmuc, was capable of inducing a F. hepatica-specific immune response and we evaluated its potential to protect animals from infection. We show that T cell lymphocytes from infected animals recognise a synthetic peptide derived from Fhmuc, and that this peptide induces Th1-like immune responses specific for F. hepatica with a high production of IFNγ. Finally, we present preliminary data indicating that animals treated with Fhmuc exhibited reduced liver damage compared to non-immunised animals and that this protection was associated with an increase of the production of IFNγ/IL-5 by splenocytes and with a recruitment of B and T lymphocytes in the peritoneum, as well as eosinophils and mature dendritic cells (DCs).
Material and methods
Mice
Six- to 8-week-old female C57BL/6 mice were obtained from DILAVE Laboratories (Uruguay). Animals were kept in the animal house (URBE, School of Medicine, UdelaR, Uruguay) with water and food supplied ad libitum. Mouse experiments were carried out in accordance with strict guidelines from the National Committee on Animal Research (Comisión Nacional de Experimentación Animal, CNEA, http://www.cnea.org.uy/, National Law 18.611, Uruguay). Procedures involving animals were approved by the Universidad de la República’s Committee on Animal Research (Comisión Honoraria de Experimentación Animal, CHEA Protocol Number 071140-000443-10).
Fhmuc peptide
A 66 amino acid peptide of a F. hepatica mucin like-protein (corresponding to a part of predicted protein of contig FH00023), named Fhmuc, was chemically synthesised by Peptide 2.0 Inc. (VA, USA). FhmucL (long) and FhmucS (short) isoforms (Cancela et al. 2015) were aligned with ClustalW and the sequence between residues 87 and 172 that shares high homology between both isoforms, was selected for this study. The amino acid sequence of this peptide, Fhmuc, is H2N-VSSDASTTSTTMTARSSSASATASSETRAPSSTMTTQNASTTSGSVRLPIQTTRCILLFIFGVAFF-COOH. Further sequence analyses were performed using Signal P 4.1 (http://www.cbs.dtu.dk/services/SignalP) for detection of a N-terminal signal peptide; NetOGlyc 4.0 (http://www.cbs.dtu.dk/services/NetOGlyc) for prediction of O-glycosylated sites; GPI-SOM (http://gpi.unibe.ch/) and big-PI predictor (http://mendel.imp.ac.at/sat/gpi/gpi_server.html) for prediction of glycosylphosphatidyldinositol (GPI) sites; and DAS-TMfilter (http://mendel.imp.ac.at/sat/DAS/DAS.html) for prediction of transmembrane domains.
Preparation of excretion/secretion products from F. hepatica (FhESP)
Live adult worms of F. hepatica were obtained from the bile ducts of bovine livers and then washed for 1 h at 37 °C with PBS (pH 7.4). Flukes were incubated at 37 °C for 3 h (one worm/2 mL) in RPMI-1640 with glutamine (PAA Laboratories, Austria) supplemented with 2 % glucose, 30 mM HEPES, 100 U/mL penicillin, and 100 mg/mL streptomycin (Sigma-Aldrich, MO, USA). Then, the supernatant was centrifuged (10,000g, 30 min, 4 °C), concentrated using a high-flow YM-10 membrane filter (Millipore-Amicon Corp., MA, USA), and stored at −20 °C until use. Endotoxins were removed using Detoxi-Gel Endotoxin Removing Gel (Thermo Fisher Scientific Inc., IL, USA) according to the instructions of the manufacturer. The protein concentration of parasitic lysates was measured using a bicinchoninic acid assay (Sigma-Aldrich, MO, USA). The endotoxin levels were determined using the Pyrochrome Limulus Amebocyte Lysate kit (Associates of Cape Cod Inc., MA, USA).
Infections and cell culture
A group of five animals were orally infected with ten F. hepatica metacercariae (Baldwin Aquatics, OR, USA) per animal. Following 3 weeks of infection, mice were bled and necropsied, and the livers, spleens, hepatic-draining lymph nodes (HLNs) and peritoneal exudates cells (PECs) were removed. Splenocytes or PECs (2.5–5 × 106 cells/mL) were cultured in complete medium consisting of RPMI-1640 with glutamine supplemented with 10 % heat-inactivated foetal bovine serum (FBS), 50 μM 2-mercaptoethanol, 100 U/mL penicillin, and 100 mg/mL streptomycin for 72 h in the presence or absence of Fhmuc peptide or FhESP (10 μg/mL). Secreted cytokine (IFNγ, IL-5, and IL-17) levels of culture supernatants were measured by interleukin-specific sandwich ELISA assays (BD Biosciences, NJ, USA). Antibody reactivity against Fhmuc or FhESP from obtained sera was analysed by ELISA. Then, hepatic damage was histologically analysed, confirming >80 % tissue damage in infected animals. Naive animals were used as control group (n = 5).
T cell response in Fhmuc-immunised mice
Mice (n = 8 per group) were immunised intraperitoneally (i.p.) with Fhmuc (20 μg) or PBS (control group) in complete Freund’s adjuvant on day 0 followed by two additional injections in incomplete Freund’s adjuvant on days 14 and 28. Two weeks after the final immunisation, mice were sacrificed and the spleens, HLN, and PECs were removed. PECs were harvested by washing the peritoneal cavity with 10 mL of cold PBS. Cells were dispersed manually, centrifuged at 1000g for 5 min, and suspended (1 × 106 cells/well) in complete culture medium. Cells were incubated in 96-well plates with Fhmuc peptide or FhESP (10 μg/mL) or medium alone for 72 h at 37 °C with 5 % CO2. Secreted cytokine (IFNγ, IL-5, and IL-17) levels of culture supernatants were measured by interleukin-specific sandwich ELISA assays (BD Biosciences, NJ, USA).
Evaluation of antibody reactivity
Mice (n = 8) were immunised i.p. with Fhmuc (20 μg) or PBS (control group) in complete Freund’s adjuvant on day 0 followed by two additional injections in incomplete Freund’s adjuvant on days 14 and 28. Bleedings were carried out at day 42, and sera reactivity was analysed by ELISA. Briefly, 96-well microtiter plates (Nunc, Denmark) were coated with Fhmuc or FhESP (1 μg/well) in 50 mM carbonate buffer (pH 9.6) overnight at 4 °C. After blocking with PBS containing 1 % gelatin, wells were washed three times with PBS containing 0.1 % Tween 20. Then, serially diluted sera in buffer (PBS containing 0.1 % Tween 20 and 0.5 % gelatin) were added to each well and incubated for 1 h at 37 °C. Wells were washed three times as before, and then treated with goat anti-mouse polyvalent IgM or IgG conjugated to peroxidase (Sigma-Aldrich, MO, USA) for 1 h at 37 °C prior to the addition of the substrate o-phenylenediamine-H2O2. Plates were read photometrically at 492 nm in an ELISA auto-reader (LabSystems Multiskan MS, Thermo Scientific). The negative control consisted of sera from mice injected with PBS in adjuvant diluted 100-fold.
Vaccination experiments
For protection assays, two groups of at least eight mice per group each were used. Animals were vaccinated i.p. with Fhmuc (20 μg) or PBS (control group) in complete Freund’s adjuvant on day 0 followed by two additional injections in incomplete Freund’s adjuvant on days 14 and 28. On day 42, mice were infected with ten metacercariae/mouse. Following 3 weeks of infection, mice were bled and necropsied, and the livers, spleens, HLN, and PECs were removed. Splenocytes or PECs (2.5–5 × 106 cells/mL) were cultured in complete medium for 72 h in the presence or absence of Fhmuc peptide or FhESP (10 μg/mL). Secreted cytokine (IFNγ, IL-5, and IL-17) levels of culture supernatants were measured by interleukin-specific sandwich ELISA assays (BD Biosciences, NJ, USA). Antibody reactivity against Fhmuc or FhESP from obtained sera was analysed by ELISA. Livers were also removed at 3 weeks post-infection and embedded in paraffin to perform histological analysis. Paraffin sections were cut from the livers and stained with haematoxylin-eosin. Liver damage in multiple sections of hepatic tissues representative of the organ was characterised according to the percentage (%) of affected area and classified into three categories: low damage (between 0 and 5 % damaged tissue), medium (up to 50 % damaged tissue), and high (more than 60 % damaged tissue). The affected area was taken into consideration if lymphocyte infiltration (LI), hydropic degeneration (HD), or necrosis (N) were detected. Liver sections from uninfected and non-vaccinated infected animals served as control groups.
Cell analysis by flow cytometry
Splenocytes and PECs from Fhmuc- or PBS-immunised or vaccinated mice were washed twice with PBS containing 2 % FBS (PAA Laboratories, Austria) and 0.1 % sodium azide (Sigma-Aldrich, MO, USA). Cells were stained with antibodies to identify B and T cells [anti-CD3 (17A2), anti-CD4 (RM4-5), anti-CD8α (53-6.7), and anti-CD19 (eBio1 D3)]; natural killer (NK) cells [anti-NK1.1-PE (PK136), anti-CD69-FITC (H1.2F3), and anti-CD49b-APC (DX5)] dendritic cells or macrophages [anti-CD11b (M1/70), anti-CD11c (N418), anti-CD40 (HM40-3), anti-MHC II (m5/114.15.2), I-A/I-E (2G9), anti-F4/80 (BM8), anti-CD80 (16-10A1), and ant-CD86 (GL1)]; and monocytes or granulocytes [anti-CD11b (M1/7c0), anti-Ly-6G (RB6-8C5), anti-Ly-6C (HK1.4), and anti-Siglec-F (E50-2440)]. Cells were washed twice with PBS containing 2 % FBS and 0.1 % sodium azide, and fixed with 1 % formaldehyde. Cell populations were analysed using a CyAn ADP Analyzer (Beckman Coulter, CA, USA). Antibodies were obtained from eBioscience (CA, USA) or from BD Biosciences (CA, USA).
Statistical analysis
An unpaired parametric t test performed on GraphPad Prism version 5.01 was used for all statistical comparisons; P values of <0.05 or <0.01 were considered to be statistically significant, depending on the experiment.
Results
Biochemical characteristics of Fhmuc
Fhmuc is a 66 amino acid peptide from a F. hepatica mucin-like protein transcript (contig FH00023) recently identified in the NEJ stage of the parasite (Cancela et al. 2010). By analysis of F. hepatica juvenile expressed sequence tags (EST), these authors identified two groups of mucin-like transcripts, FhmucL and FhmucS (Cancela et al. 2015). which share a high degree of homology and are primarily distinguished by a 22-residue amino acid insertion in FhmucS (Fig. 1). Secreted mucins or mucin-like proteins are characterised by an N-terminal signal peptide, Ser/Thr-rich domains (potentially highly O-glycosylated) called tandem repeats, and a C-terminal domain. In F. hepatica, the predicted Fhmuc protein transcript has an N-terminal 20-residue signal peptide with a typical hydrophobic core. The signal peptidase cleavage site is most likely located after the Thr residue preceding the Glu in position 1 of the mature protein, as predicted by the Signal P 4.1 server (http://www.cbs.dtu.dk/services/SignalP). The sequence immediately following this cleavage site corresponds to the Ser/Thr-rich region. FhmucL possesses two tandem repeats in which all hydroxylated residues in the Ser/Thr-rich region (62 Ser/Thr) are predicted to be O-glycosylated by the NetOGlyc 4.0 server (http://www.cbs.dtu.dk/services/NetOGlyc). Finally, there is a C-terminal region of 22 residues, composed primarily of hydrophobic amino acids (Fig. 1). No glycosylphosphatidylinositol (GPI) modification sites or transmembrane domains were found using different prediction softwares (GPI-SOM server at http://gpi.unibe.ch/, big-PI predictor at http://mendel.imp.ac.at/sat/gpi/gpi_server.html, DAS-TMfilter at http://mendel.imp.ac.at/sat/DAS/DAS.html). Taking into account the high degree of homology shared between both Fhmuc isoforms, a shorter peptide comprising the sequence between residues 87 and 172, encompassing most of the second tandem repeat region and the C-terminal domain, of FhmucL was selected for this study (Fig. 1), and will be referred as Fhmuc.
Predicted biochemical characteristics of Fhmuc. a Amino acid sequences of the two predicted isoforms FhmucL (long) and FhmucS (short). The alignments were performed by ClustalW. Gaps have been inserted to maximise sequence identity. Fully conserved residues are marked with asterisks, residues with strongly similar properties with colons and weakly similar properties with dots. The sequences are divided into three domains: signal peptide, the Ser/Thr-rich region, and the C-terminus characterised by the presence of four phenylalanine residues. The sequence corresponding to the Fhmuc peptide used in this study is marked in bold and represented with an arrow
Fasciola hepatica specific-immune response recognises the Fhmuc peptide
To determine whether the immune response induced during F. hepatica infection was able to recognise the Fhmuc peptide, we analysed the reactivity of both antibodies and splenocytes from infected animals. Mice infected with ten metacercariae were killed 3 weeks post-infection. Then, hepatic damage was histologically analysed, confirming >80 % tissue damage in infected animals. Cells obtained from the spleen, PECs, and HLNs from infected animals were stimulated with excretion/secretion parasite-derived products (FhESP) or the Fhmuc peptide. The levels of IFNγ and IL-5 were evaluated in the culture supernatants as indicative of Th1 and Th2 immune response, respectively. Cells from HLN and PECs produced high levels of IL-5 but not IFNγ when stimulated with FhESP (Fig. 2a). On the other hand, spleen cells produced both IL-5 and IFNγ when stimulated with FhESP. However, when splenocytes were incubated with Fhmuc, they produced high levels of IFNγ but not IL-5 (Fig. 2b). No detectable reactivity to the different antigens was observed in cells from uninfected mice (not shown).
Antigenic and immunogenic properties of the Fhmuc peptide. a F. hepatica-induced cellular adaptive immune response recognises the Fhmuc peptide. Splenocytes, HLN cells, and PECs from mice infected with ten metacercariae were cultured either with Fhmuc or FhESP (10 μg/mL). After 3 days of culture, INFγ and IL-5 levels were quantified by ELISA. b FhESP-specific antibodies recognize the Fhmuc peptide. The reactivity of sera from infected animals was evaluated on plates coated with Fhmuc or FhESP (10 μg/mL). c Immunisation with Fhmuc induces a cellular immune response that recognises F. hepatica products. INFγ and IL-5 production by splenocytes, HLN cells and PECs from Fhmuc-immunised animals stimulated with Fhmuc or FhESP (10 μg/mL) for three days. d Fhmuc-specific antibodies do not cross-react with the Fhmuc peptide. The reactivity of sera from Fhmuc-immunised mice was evaluated by ELISA on plates coated with Fhmuc or FhESP (10 μg/mL). Results are expressed as the mean values (±SD, indicated by error bars) obtained from cells incubated with Fhmuc or FhESP normalised to the mean values obtained from cells incubated in medium alone, from three independent experiments. Asterisks indicate statistically significant differences (p < 0.01) with respect to the medium alone control
The reactivity of sera from these animals was evaluated by an ELISA-like assay using plates coated either with FhESP or Fhmuc. These animals produced high titres of FhESP-specific antibodies (Fig. 1c). Although IgG antibodies reactive to FhESP were observed, specific IgM antibodies were detected at higher titres. Interestingly, IgG antibodies from infected animals recognised the Fhmuc peptide (Fig. 1c).
Fhmuc immunisation induces an adaptive cellular immune response to F. hepatica-derived products
After verifying that F. hepatica infection induced a specific cellular and humoral immune response capable of recognising Fhmuc, we investigated whether this peptide was able to induce an immune response capable of reacting to parasite-derived molecules. As depicted in Fig. 2c, splenocytes, PECs, and HLN cells from immunised mice produced high levels of IFNγ following stimulation FhESP. Unexpectedly, we observed lower levels of reactivity with the Fhmuc peptide. Low levels of IL-5 were detected on the cell cultures that were not significantly different from the control group (Fig. 2c). IL-17 was not detected in either of the culture conditions (data not shown).
Next, the capacity of generated antibodies in Fhmuc-immunised animals to recognise secreted-excreted parasite components was evaluated by ELISA using plates coated either with Fhmuc peptides or FhESP. Immunised animals had high titres of Fhmuc-specific IgG antibodies that did not specifically recognise FhESP (Fig. 2d). IgM specific-antibodies were not detected.
Animals vaccinated with Fhmuc have reduced liver damage after infection
Taking into account that immunisation with Fhmuc induced T cell responses specific to parasite products, we investigated whether this adaptive immune response could mediate protection from parasite-caused damage (Fig. 3). Approximately 60 % of Fhmuc-vaccinated animals exhibited medium damage levels in the liver, compared to 30 % for the control group, while the opposite trend was observed for the group presenting a higher level of liver damage (Fig. 3b). Half of the livers from the control group contained at least an adult fluke when analysed by microscopy. No parasites were identified in Fhmuc-vaccinated mice. In addition, the Fhmuc-vaccinated mice exhibited significantly higher levels of protection based on liver damage, compared to the PBS-treated control group (Fig. 3c).
Fhmuc-vaccinated animals have reduced liver damage. a Analyses of the liver damage from F. hepatica-infected animals. Liver tissue sections stained with haematoxylin from infected-mice immunised with Fhmuc (20 μg/mouse) or PBS. Liver damage was considered as the percentage of tissue area with lymphocyte infiltration (LI), necrosis (N), or hydropic degeneration (HD). Representative tissue sections were selected showing (I) healthy tissue; (II) 5 %, (III) 20 %, and (IV) 50 % damage represented by lymphocyte infiltration; (V) 100 % damage with lymphocyte infiltration, necrosis, and hydropic degeneration; and (VI) identification of flukes (Fh). The bar corresponds to 50 μm. b Percentage of Fhmuc-vaccinated or control animals with different level of liver damage. Results of two individual experiments are shown. Asterisks represent statistically significant differences (*p < 0.05 or **p < 0.01). c Levels of protection from liver damage in Fhmuc-vaccinated and control mice. The protection was calculated according to the liver damage (as calculated in a) attributed to Fhmuc-vaccinated and control mice, in relation to damage of infected animals that did not received any treatment
Vaccination with Fhmuc favours the production of higher levels of IFNγ/IL-5 and the recruitment of eosinophils, mature dendritic cells, as well as T and B cells in the peritoneum of infected animals
Next, we evaluated the immune response induced in the protected animals. Splenocytes and PECs from vaccinated animals stimulated with FhESP produced lower levels of IL-5 compared to control animals (Fig. 4a) favoring a greater IFNγ/IL-5 ratio (Fig. 4b). Vaccination with Fhmuc induced the production of specific IgG antibodies that were not detected in control (PBS) mice (Fig. 4c). On the other hand, anti-FhESP specific IgG antibodies were detected both in Fhmuc- and PBS-vaccinated animals, with no differences observed in the titre of anti-FhESP antibodies from either group (Fig. 4d).
PECs from animals vaccinated with Fhmuc produce greater levels of IFNγ/IL-5 when stimulated with parasite products than control mice. a INFγ and IL-5 production by splenocytes, HLN cells, and PECs from infected-mice previously vaccinated with Fhmuc (20 μg/mouse) or PBS and challenged with ten metacercariae. Cells were incubated with Fhmuc or FhESP (10 μg/mL) for 3 days. Cytokines were quantified by ELISA. b IFNγ/IL-5 ratio produced by FhESP-stimulated cells from the spleen, PECs, or HLN cells from mice treated as explained in a. Results are expressed as the mean values (±SD, indicated by error bars) from three independent experiments. Asterisks indicate statistically significant differences (p < 0.01) with respect to the medium alone control. c Reactivity of sera from Fhmuc-vaccinated animals. Mice were vaccinated as described in a and bled 3 weeks post-infection. Antibody reactivity was evaluated on plates coated with Fhmuc or FhESP (1 μg/well)
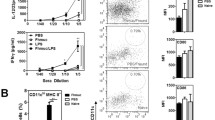
We also studied the presence of different immune cell types in protected animals by flow cytometry. The percentage levels of CD11b+, CD11b+ F4/80+ MHCII+, CD11b+/Ly6G+ Ly6C+, CD11b+/Ly6G− Ly6C+, or NK1.1+ CD49b+ were not significantly different in the spleens or PECs of Fhmuc-vaccinated mice compared to control mice (Fig. 5). However, CD11c+ cells in the peritoneum expressed higher levels of MHC class II, indicating that recruitment of mature DCs was favoured in Fhmuc-vaccinated mice (Fig. 5a). On the other hand, PECs from Fhmuc-vaccinated animals exhibited higher levels of CD11b+ Siglec-F+ cells, suggesting that more eosinophils are present in the peritoneal cavity of these mice compared to controls (Fig. 5a). Finally, the levels of CD3+ CD19− and CD19+ CD3− cells were also increased in Fhmuc-vaccinated mice, indicating a recruitment of B and T cells to the peritoneum favoured by Fhmuc-vaccination (Fig. 5b). Strikingly, the differences in the percentages of the described populations found in Fhmuc-immunised mice compared to the control group were not observed in absence of infection (Supplementary Fig. 1). The presence of greater levels of eosinophils, T or B cells, and mature DCs in the peritoneum of Fhmuc-vaccinated animals could be associated with the lower levels of liver damage found in these mice.
Immunophenotyping of lymphoid or myeloid cells from Fhmuc-vaccinated mice. Immunophenotype of splenocytes and PECs from infected mice previously vaccinated with Fhmuc (20 μg/mouse) or PBS. Myeloid (a) or lymphoid (b) cells were stained with different fluorochrome-conjugated specific antibodies and analysed by flow cytometry. Thirty thousand events were collected and gated on forward scatter (FSC) vs side scatter (SSC) dot plot. Results are shown as the percentage of cells in the spleen expressed as the mean value of eight replicates (±SD, indicated by error bars) and are representative of two different experiments. Asterisks represent statistically significant differences (*p < 0.05 or **p < 0.01)
Discussion
In this work, we propose the use of a F. hepatica vaccine that targets immature flukes since they cause the most severe damage and pathology of the liver in the host. We evaluated the immunological properties of a 66 amino acid peptide derived from putative secreted mucin-like proteins (FhmucS and FhmucL) that are over-expressed in the infective stage of the parasite (Cancela et al. 2015). Synthetic peptide-based vaccines seem to be a promising approach to treating parasite infections (Rojas-Caraballo et al. 2014) since they may induce an antigen-specific immune response, are safe, highly pure, endotoxin-free and inexpensive. They can also be composed of various epitopes from different antigens and integrate T cells and B cells epitopes into one antigenic formulation.
The recognition of the Fhmuc peptide by both the humoral and cellular immune system following F. hepatica infection indicates that antibodies and T cell reactivity induced during the infection recognise the Fhmuc peptide, and prompted us to evaluate whether this peptide could induce an immune response against the parasite. Strikingly, when the Fhmuc peptide was administered in Freund’s adjuvant, only the cellular adaptive immune response cross-reacted with parasite antigens since the Fhmuc-specific IgG antibodies did not recognise the parasite-derived products. On the other hand, the T lymphocytes primed in vivo with Fhmuc produced high levels of IFNγ but not IL-5 when stimulated with parasite-derived antigens. Unexpectedly, we observed lower levels of reactivity with the Fhmuc peptide, suggesting that T cell epitopes present in the molecules secreted by adult worms better stimulate IFNγ production by Fhmuc-T cell primed cells. Interestingly, high IFNγ/IL-5 ratios were maintained in Fhmuc-vaccinated mice since they produced lower levels of IL-5 when splenocytes and PECs were cultured with excretion/secretion parasite products.
The increase in IFNγ/IL-5 ratio could be associated with the fact that Fhmuc-vaccinated animals presented significantly lower levels of hepatic damage when compared to the control group. Furthermore, it seems that the control group receiving PBS in Freund’s adjuvant exhibited a background level of protection. This could be explained by the immunostimulatory properties of this adjuvant. Indeed, apart from its capacity to retain and slowly release the antigens, Freund’s adjuvant has been demonstrated to activate antigen-presenting cells (including DCs and macrophages) through the recognition of TLR2 ligands present in the Mycobacterium tuberculosis component (Lim 2003). In this sense, other adjuvants have also shown some degree of protection in F. hepatica experimental animals (Rojas-Caraballo et al. 2014). Similar to our results, total F. hepatica antigens mixed with Freund’s adjuvant also protected rats from infection, while the group treated with PBS plus Freund adjuvant presented a background but detectable level of protection (Cervi et al. 2004).
Vaccinated animals did not have a complete absence of liver damage, probably due to the fact they were infected with a high parasite dose. Thus, the IFNγ levels were not significantly different in HLN cells between Fhmuc-vaccinated and control mice. Indeed, resistance to liver fluke infection in sheep was shown to be associated with a type 1 cytokine response in HLN (Pleasance et al. 2011). Moreover, it has been proposed that the induction of a Th1 immune response could protect the host from infection (Toet et al. 2014) and bystander co-infections by down-regulating the induced Th2 regulatory immunity (Garza-Cuartero et al. 2014). In agreement with these facts, protection induced by many vaccines against helminths has been associated with high IFNγ and TNFα production (Cardoso et al. 2008). Evidence demonstrating that antibodies are involved in helminth killing and clearance has been reported. For instance, antibody-dependent cell cytotoxity (ADCC) mediated by macrophages has been described (Piedrafita et al. 2007). However, we could not detect any difference between the FhESP-specific antibody titres induced by Fhmuc-vaccinated and control mice, possibly masked by the immune response induced by the infection itself, suggesting that protection could be mediated by immune cells or their secreted cytokines.
There are very few reports evaluating the protective capacity of peptide-based vaccines against this parasite. One recent study evaluated a variety of peptides carrying B or T cells epitopes derived from previously examined candidate proteins, mostly cathepsins (Rojas-Caraballo et al. 2014). One of the peptides that showed the highest level of protection induced high levels of IFNγ, suggesting that Th1 cytokines could be exploited to measure vaccination effectiveness. Strikingly, this peptide, corresponding to a B cell epitope from the cathepsin B3, shares a high degree of homology with the sequence of Fhmuc (45 % of amino acids are identical and 20 % of similar amino acids; see Supplementary Fig. 2).
Fhmuc vaccination was also associated with the recruitment of eosinophils, B and T cells in the peritoneum. Although eosinophils have been reported to be correlated with helminth protection (Van Milligen et al. 1999). their role is controversial. Studies using murine hosts deficient in eosinophils failed to exhibit overall differences in helminth parasite burden (Swartz et al. 2006). Likewise, the role of CD3+ T cells in protection against F. hepatica infection remains to be clearly established. We have previously shown that infected mice have significant lower levels of T cells in the peritoneum (Noya et al. 2014). In the same line, susceptible sheep show reductions in T-lymphocyte proliferation during F. hepatica infection (Zimmerman et al. 1983).
Vaccination of mice with Fhmuc was also associated with the presence of higher levels of MHC class II expression on peritoneal CD11c+ cells. DCs are potent antigen presenting cells that possess the ability to stimulate naive T cells. We and others have reported that peritoneal DCs from infected mice are characterised by a semi-mature phenotype associated with an important decrease in MHC class II expression and an up-regulation in the co-stimulatory molecules CD80 and CD86 (Noya et al. 2014; Walsh et al. 2009). In this sense an increase of MHC class II expression on DCs induced by Fhmuc-vaccination could favour T cell priming by increasing antigen presentation (Steinman 2012) and promote effective anti-parasite immunity.
In conclusion, although the protection levels detected in Fhmuc-vaccinated animals were modest, we show that a mucin-like peptide generates a parasite-specific cellular immune response and promotes the recruitment of certain immune cell populations into the peritoneum that was associated with protection in terms of hepatic lesion severity. A further comprehensive analysis of its protective properties is needed to define the use of this peptide.
Abbreviations
- DCs:
-
Dendritic cells
- HLN:
-
Hepatic lymph node
- MHC:
-
Major histocompatibility complex
References
Buscaglia C, Campo V, Frasch A, Di Noia J (2006) Trypanosoma cruzi surface mucins: host-dependent coat diversity. Nat Rev Microbiol 4:229–236
Cancela M, Ruétalo N, Dell’Oca N, da Silva E, Smircich P, Rinaldi G, Roche L, Carmona C, Alvarez-Valín F, Zaha A, Tort J (2010) Survey of transcripts expressed by the invasive juvenile stage of the liver fluke Fasciola hepatica. BMC Genomics 11:227
Cancela M, Santos GB, Carmona C, Ferreira HB, Tort JF, Zaha A (2015) Fasciola hepatica mucin-encoding gene: expression, variability and its potential relevance in host-parasite relationship. Parasitology 1–9. doi:10.1017/S0031182015001134
Cardoso FC, Macedo GC, Gava E, Kitten GT, Mati VL, de Melo AL, Caliari MV, Almeida GT, Venancio TM, Verjovski-Almeida S, Oliveira SC (2008) Schistosoma mansoni tegument protein Sm29 is able to induce a Th1-type of immune response and protection against parasite infection. PLoS Negl Trop Dis 2(10):e308. doi:10.1371/journal.pntd.0000308
Cervi L, Borgonovo J, Egea M, Chiapello L, Masih D (2004) Immunization of rats against Fasciola hepatica using crude antigens conjugated with Freund’s adjuvant or oligodeoxynucleotides. Vet Immunol Immunopathol 97(1-2):97–104
Claridge J, Diggle P, McCann C, Mulcahy G, Flynn R, McNair J, Strain S, Welsh M, Baylis M, Williams D (2012) Fasciola hepatica is associated with the failure to detect bovine tuberculosis in dairy cattle. Nat Commun 3:doi:10.1038/ncomms1840
Donnelly S, Stack S, O’Neill S, Sayed A, Williams D, Dalton J (2008) Helminth 2-Cys peroxiredoxin drives Th2 responses through a mechanism involving alternatively activated macrophages. FASEB J 22:4022–4032
Fairweather I (2009) Triclabendazole progress report, 2005-2009: an advancement of learning? J Helminthol 83:139–150
Fairweather I (2011) Reducing the future threat from (liver) fluke: realistic prospect or quixotic fantasy? Vet Parasitol 180:133–143
Flynn R, Mulcahy G (2008) The roles of IL-10 and TGF-beta in controlling IL-4 and IFN-gamma production during experimental Fasciola hepatica infection. Int J Parasitol 38:1673–1680
Flynn R, Mannion C, Golden O, Hacariz O, Mulcahy G (2007) Experimental Fasciola hepatica infection alters responses to tests used for diagnosis of bovine tuberculosis. Infect Immun 75:1373–1381
Garza-Cuartero L, Garcia-Campos A, Zintl A, Chryssafidis A, O’Sullivan J, Sekiya M, Mulcahy G (2014) The worm turns: trematodes steering the course of co-infections. Vet Pathol 51(2):385–392. doi:10.1177/0300985813519655
Hillyer GV (2005) Fasciola antigens as vaccines against fascioliasis and schistosomiasis. J Helminthol 79(3):241–247
Keiser J, Engels D, Büscher G, Utzinger J (2005) Triclabendazole for the treatment of fascioliasis and paragonimiasis. Expert Opin Investig Drugs 14:1513–1526
Khan M, Sajid M, Riaz H, Ahmad N, He L, Shahzad M, Hussain A, Khan M, Iqbal Z, Zhao J (2013) The global burden of fasciolosis in domestic animals with an outlook on the contribution of new approaches for diagnosis and control. Parasitol Res 112:2421–230
Lim SK (2003) Freund adjuvant induces TLR2 but not TLR4 expression in the liver of mice. Int Immunopharmacol 3(1):115–118
Loukas A, Hintz M, Linder D, Mullin N, Parkinson J, Tetteh K, Maizels R (2000) A family of secreted mucins from the parasitic nematode Toxocara canis bears diverse mucin domains but shares similar flanking six-cysteine repeat motifs. J Biol Chem 275:39600–39607
Maizels R (2013) Toxocara canis: molecular basis of immune recognition and evasion. Vet Parasitol 193:365–374
Maizels R, Tetteh K, Loukas A (2000) Toxocara canis: genes expressed by the arrested infective larval stage of a parasitic nematode. Int J Parasitol 30:495–508
McManus D, Dalton J (2006) Vaccines against the zoonotic trematodes Schistosoma japonicum, Fasciola hepatica and Fasciola gigantica. Parasitology 133:S43–S61
Molina-Hernandez V, Mulcahy G, Perez J, Martinez-Moreno A, Donnelly S, O’Neill SM, Dalton JP, Cwiklinski K (2015) Fasciola hepatica vaccine: we may not be there yet but we’re on the right road. Vet Parasitol 208(1-2):101–111. doi:10.1016/j.vetpar.2015.01.004
Noya V, Rodríguez E, Cervi L, Giacomini C, Brossard N, Chiale C, Carmona C, Freire T (2014) Modulation of dendritic cell maturation by Fasciola hepatica: Implications of glycans and mucins for vaccine development. J Vaccines Vaccin 5(4):233. doi:10.4172/2157-7560.1000233
O’Neill S, Brady M, Callanan J, Mulcahy G, Joyce P, Mills K, Dalton J (2000) Fasciola hepatica infection downregulates Th1 responses in mice. Parasite Immunol 22:147–155
Perrin C, Lepesant J, Roger E, Duval D, Fneich S, Thuillier V, Alliene J, Mitta G, Grunau C, Cosseau C (2013) Schistosoma mansoni mucin gene (SmPoMuc) expression: epigenetic control to shape adaptation to a new host. PLoS Pathog 9:e1003571
Piedrafita D, Estuningsih E, Pleasance J, Prowse R, Raadsma HW, Meeusen EN, Spithill TW (2007) Peritoneal lavage cells of Indonesian thin-tail sheep mediate antibody-dependent superoxide radical cytotoxicity in vitro against newly excysted juvenile Fasciola gigantica but not juvenile Fasciola hepatica. Infect Immun 75(4):1954–1963. doi:10.1128/IAI.01034-06
Piedrafita D, Spithill T, Smith R, Raadsma H (2010) Improving animal and human health through understanding liver fluke immunology. Parasite Immunol 32:572–581
Pleasance J, Wiedosari E, Raadsma HW, Meeusen E, Piedrafita D (2011) Resistance to liver fluke infection in the natural sheep host is correlated with a type-1 cytokine response. Parasite Immunol 33(9):495–505. doi:10.1111/j.1365-3024.2011.01305.x
Roger E, Gourbal B, Grunau C, Pierce R, Galinier R, Mitta G (2008) Expression analysis of highly polymorphic mucin proteins (Sm PoMuc) from the parasite Schistosoma mansoni. Mol Biochem Parasitol 157:217–227
Rojas-Caraballo J, Lopez-Aban J, Perez del Villar L, Vizcaino C, Vicente B, Fernandez-Soto P, del Olmo E, Patarroyo MA, Muro A (2014) In vitro and in vivo studies for assessing the immune response and protection-inducing ability conferred by Fasciola hepatica-derived synthetic peptides containing B- and T-cell epitopes. PLoS One 9(8):e105323. doi:10.1371/journal.pone.0105323
Rojo-Vázquez F, Meana A, Valcárcel F, Martínez-Valladares M (2012) Update on trematode infections in sheep. Vet Parasitol 189:15–38
Steinman RM (2012) Decisions about dendritic cells: past, present, and future. Annu Rev Immunol 30:1–22. doi:10.1146/annurev-immunol-100311-102839
Swartz JM, Dyer KD, Cheever AW, Ramalingam T, Pesnicak L, Domachowske JB, Lee JJ, Lee NA, Foster PS, Wynn TA, Rosenberg HF (2006) Schistosoma mansoni infection in eosinophil lineage-ablated mice. Blood 108(7):2420–2427. doi:10.1182/blood-2006-04-015933
Theodoropoulos G, Hicks SJ, Corfield AP, Miller BG, Carrington SD (2001) The role of mucins in host-parasite interactions: Part II - helminth parasites. Trends Parasitol 17(3):130–135
Toet H, Piedrafita DM, Spithill TW (2014) Liver fluke vaccines in ruminants: strategies, progress and future opportunities. Int J Parasitol 44(12):915–927. doi:10.1016/j.ijpara.2014.07.011
Van Milligen FJ, Cornelissen JB, Bokhout BA (1999) Protection against Fasciola hepatica in the intestine is highly correlated with eosinophil and immunoglobulin G1 responses against newly excysted juveniles. Parasite Immunol 21(5):243–251
Walsh K, Brady M, Finlay C, Boon L, Mills K (2009) Infection with a helminth parasite attenuates autoimmunity through TGF-beta-mediated suppression of Th17 and Th1 responses. J Immunol 183:1577–1586
Zimmerman GL, Kerkvliet NI, Brauner JA, Cerro JE (1983) Modulation of host immune responses by Fasciola hepatica: responses by peripheral lymphocytes to mitogens during liver fluke infections of sheep. J Parasitol 69(3):473–477
Acknowledgments
We are especially thankful to the abattoirs ‘Frigorífico Carrasco’ and ‘Frigrorífico Sarubbi’ for their help with the collection of worms.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Funding
This work was supported by grants from the Agencia Nacional de Investigación e Innovación (PR-FCE-2009-1-2782, ANII, Uruguay) and Comisión Sectorial de Investigación Científica (CSIC, Universidad de la República, Uruguay). VN and ER were supported by CSIC and ANII, respectively.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
Supplementary Figure 1. Immunophenotyping of lymphoid or myeloid cells from Fhmuc-immunised animals in absence of infection. Immunophenotype of splenocytes and PECs from Fhmuc-immunised (20 μg/mouse) or PBS-treated mice. The immunisation was i.p. on days 0 in Complete Freund’s adjuvant followed by two additional injections at days 14 and 28 in Incomplete Freund’s adjuvant. Three weeks after infection, animals were sacrificed and spleen, HLN or PECs were removed. Cells were stained with different fluorochrome-conjugated specific antibodies and analysed by flow cytometry. Different myeloid (A) or lymphoid (B) cell populations were evaluated. Thirty thousand events were collected and gated on FSC vs SSC dot plot. Results are shown as the percentage of cells in the spleen expressed as the mean value of eight replicates (±SD, indicated by error bars) and are representative of two different experiments. Asterisks (*) represent statistically significant differences (p < 0.05).
Supplementary Figure 2. Amino acid sequence alignment of the Fhmuc peptide and the B5 peptide from cathepsin B. Sequence alignment of the Fhmuc peptide and the B5 peptide from Cathepsin B3 (GenBank accession number ABU62925.1 (sequence ISEIRDQSSTSSTWAVSSAS). The alignment was performed by ClustalW. Gaps have been inserted to maximise amino acid identity. Fully conserved residues are marked with (*), residues with strongly similar properties with (:) and weakly similar properties with (.). The B5 peptide sequence was reported by Rojas-Caraballo et al. (2014). (PDF 217 kb)
Rights and permissions
About this article
Cite this article
Noya, V., Brossard, N., Berasaín, P. et al. A mucin-like peptide from Fasciola hepatica induces parasite-specific Th1-type cell immunity. Parasitol Res 115, 1053–1063 (2016). https://doi.org/10.1007/s00436-015-4834-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-015-4834-z