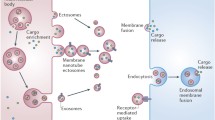
Abstract
Parasite-host cell interaction can be modulated by a dynamic communication between extracellular vesicles (EVs). They should play key roles in cell-cell communications transferring biomolecules (miRNA, proteins, soluble factors) from one cell to another cell. While many names have been used to denominate EVs, a better comprehension to understand these vesicles is raised when we classify it according to biogenesis: originated from multivesicular bodies, named exosomes, and from plasmatic membranes, denominated microvesicles. Here, we have reviewed EV participation during the protozoan-host cell interaction and reinforced the differences and similarities between exosomes and microvesicles, suggesting different intracellular routes and functions. We also discussed perspectives to study EVs and the role of EVs in diagnosis and chemotherapies of infectious diseases.


Similar content being viewed by others
References
Aline F, Bout D, Amigorena S, Roingeard P, Dimier-Poisson I (2004) Toxoplasma gondii antigen-pulsed-dendritic cell-derived exosomes induce a protective immune response against T. gondii infection. Infect Immun 72:4127–4137
Barteneva NS, Maltsev N, Vorobjev IA (2013) Microvesicles and intercellular communication in the context of parasitism. Front Cell Infect Microbiol 3:49
Bayer-Santos E, Aguilar-Bonavides C, Rodrigues SP, Cordero EM, Marques AF, Varela-Ramirez A, Choi H, Yoshida N, da Silveira JF, Almeida IC (2012) Proteomic analysis of Trypanosoma cruzi secretome: characterization of two populations of extracellular vesicles and soluble proteins. J Proteome Res 12:883–897
Benchimol M (2004) The release of secretory vesicle in encysting Giardia lamblia. FEMS Microbiol Lett 235:81–87
Bhatnagar S, Shinagawa K, Castellino FJ, Schorey JS (2007) Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood 110:3234–3244
Brun R, Blum J (2012) Human African trypanosomiasis. Infect Dis Clin N Am 26:261–273
Bucci C, Thomsen P, Nicoziani P, McCarthy J, van Deurs B (2000) Rab7: a key to lysosome biogenesis. Mol Biol Cell 11(2):467–480
Cestari I, Ansa-Addo E, Deolindo P, Inal JM, Ramirez MI (2012) Trypanosoma cruzi immune evasion mediated by host cell-derived microvesicles. J Immunol 188:1942–1952
Chen Y, Chan CK, Kerishnan JP, Lau YL, Wong YL, Gopinath SC (2015) Identification of circulating biomarkers in sera of Plasmodium knowlesi-infected malaria patients--comparison against Plasmodium vivax infection. BMC Infect Dis 15:49
Coltel N, Combes V, Wassmer SC, Chimini G, Grau GE (2006) Cell vesiculation and immunopathology: implications in cerebral malaria. Microbes Infect 8:2305–2316
Combes V, Coltel N, Alibert M, van Eck M, Raymond C, Juhan-Vague I, Grau GE, Chimini G (2005) ABCA1 gene deletion protects against cerebral malaria: potential pathogenic role of microparticles in neuropathology. Am J Pathol 166:295–302
da Silveira JF, Abrahamsohn PA, Colli W (1979) Plasma membrane vesicles isolated from epimastigote forms of Trypanosoma cruzi. Biochim Biophys Acta 550:222–232
de Miguel N, Lustig G, Twu O, Chattopadhyay A, Wohlschlegel JA, Johnson PJ (2010) Proteome analysis of the surface of Trichomonas vaginalis reveals novel proteins and strain-dependent differential expression. Mol Cell Proteomics 9:1554–1566
Deolindo P, Evans-Osses I, Ramirez MI (2013) Microvesicles and exosomes as vehicles between protozoan and host cell communication. Biochem Soc Trans 41:252–257
Embley TM, Hirt RP (1998) Early branching eukaryotes? Curr Opin Genet Dev 8:624–629
Garcia-Silva MR, Cabrera-Cabrera F, das Neves RF, Souto-Padron T, de Souza W, Cayota A (2014) Gene expression changes induced by Trypanosoma cruzi shed microvesicles in mammalian host cells: relevance of tRNA-derived halves. Biomed Res Int 2014:305239
Geiger A, Hirtz C, Becue T, Bellard E, Centeno D, Gargani D, Rossignol M, Cuny G, Peltier JB (2010) Exocytosis and protein secretion in Trypanosoma. BMC Microbiol 10:20
Ghildiyal M, Zamore PD (2009) Small silencing RNAs: an expanding universe. Nat Rev Genet 10:94–108
Giusti I, D’Ascenzo S, Dolo V (2013) Microvesicles as potential ovarian cancer biomarkers. Biomed Res Int 2013:703048
Goncalves MF, Umezawa ES, Katzin AM, de Souza W, Alves MJ, Zingales B, Colli W (1991) Trypanosoma cruzi: shedding of surface antigens as membrane vesicles. Exp Parasitol 72:43–53
Gottig N, Elias EV, Quiroga R, Nores MJ, Solari AJ, Touz MC, Lujan HD (2006) Active and passive mechanisms drive secretory granule biogenesis during differentiation of the intestinal parasite Giardia lamblia. J Biol Chem 281:18156–18166
Kanada M, Bachmann MH, Hardy JW, Frimannson DO, Bronsart L, Wang A, Sylvester MD, Schmidt TL, Kaspar RL, Butte MJ, Matin AC, Contag CH (2015) Differential fates of biomolecules delivered to target cells via extracellular vesicles. Proc Natl Acad Sci U S A 112(12):E1433–42
Kim DK, Lee J, Kim SR, Choi DS, Yoon YJ, Kim JH, Go G, Nhung D, Hong K, Jang SC, Kim SH, Park KS, Kim OY, Park HT, Seo JH, Aikawa E, Baj-Krzyworzeka M, van Balkom BW, Belting M, Blanc L, Bond V, Bongiovanni A Borràs FE, Buée L, Buzás EI, Cheng L, Clayton A, Cocucci E, Dela Cruz CS, Desiderio DM, Di Vizio D, Ekström K, Falcon-Perez JM, Gardiner C, Giebel B, Greening DW, Gross JC, Gupta D, Hendrix A, Hill AF, Hill MM, Nolte-'t Hoen E, Hwang do W, Inal J, Jagannadham MV, Jayachandran M, Jee YK, Jørgensen M, Kim KP, Kim YK, Kislinger T, Lässer C, Lee DS, Lee H, van Leeuwen J, Lener T, Liu ML, Lötvall J, Marcilla A, Mathivanan S, Möller A, Morhayim J, Mullier F, Nazarenko I, Nieuwland R, Nunes DN, Pang K, Park J, Patel T, Pocsfalvi G, Del Portillo H, Putz U, Ramirez MI, Rodrigues ML, Roh TY, Royo F, Sahoo S, Schiffelers R, Sharma S, Siljander P, Simpson RJ, Soekmadji C, Stahl P, Stensballe A, Stępień E, Tahara H, Trummer A, Valadi H, Vella LJ, Wai SN, Witwer K, Yáñez-Mó M, Youn H, Zeidler R, Gho YS (2015) EVpedia: a community web portal for extracellular vesicles research. Bioinformatics 31(6):933–939
Liu Q, Tuo W, Gao H, Zhu XQ (2010) MicroRNAs of parasites: current status and future perspectives. Parasitol Res 107:501–507
Mantel PY, Hoang AN, Goldowitz I, Potashnikova D, Hamza B, Vorobjev I, Ghiran I, Toner M, Irimia D, Ivanov AR, Barteneva N, Marti M (2013) Malaria-infected erythrocyte-derived microvesicles mediate cellular communication within the parasite population and with the host immune system. Cell Host Microbe 13:521–534
Marcilla A, Martin-Jaular L, Trelis M, de Menezes-Neto A, Osuna A, Bernal D, Fernandez-Becerra C, Almeida IC, Del Portillo HA (2014) Extracellular vesicles in parasitic diseases. J Extracell Vesicles 3:25040
Martinez MC, Tual-Chalot S, Leonetti D, Andriantsitohaina R (2011) Microparticles: targets and tools in cardiovascular disease. Trends Pharmacol Sci 32(11):659–665
Martin-Jaular L, Nakayasu ES, Ferrer M, Almeida IC, Del Portillo HA (2011) Exosomes from Plasmodium yoelii-infected reticulocytes protect mice from lethal infections. PLoS One 6:e26588
Montaner S, Galiano A, Trelis M, Martin-Jaular L, Del Portillo HA, Bernal D, Marcilla A (2014) The role of extracellular vesicles in modulating the host immune response during parasitic infections. Front Immunol 5:433
Nakano I, Garnier D, Minata M, Rak J (2015) Extracellular vesicles in the biology of brain tumour stem cells--Implications for inter-cellular communication, therapy and biomarker development. Semin Cell Dev Biol 40:17–26
Nantakomol D, Dondorp AM, Krudsood S, Udomsangpetch R, Pattanapanyasat K, Combes V, Grau GE, White NJ, Viriyavejakul P, Day NP, Chotivanich K (2011) Circulating red cell-derived microparticles in human malaria. J Infect Dis 203:700–706
Neves RF, Fernandes AC, Meyer-Fernandes JR, Souto-Padron T (2014) Trypanosoma cruzi-secreted vesicles have acid and alkaline phosphatase activities capable of increasing parasite adhesion and infection. Parasitol Res 113:2961–2972
Pope SM, Lasser C (2013) Toxoplasma gondii infection of fibroblasts causes the production of exosome-like vesicles containing a unique array of mRNA and miRNA transcripts compared to serum starvation. J Extracellular Vesicles 2
Raposo G, Stoorvogel W (2013) Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 200:373–383
Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak MZ (2006) Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia 20:1487–1495
Regev-Rudzki N, Wilson DW, Carvalho TG, Sisquella X, Coleman BM, Rug M, Bursac D, Angrisano F, Gee M, Hill AF, Baum J, Cowman AF (2013) Cell-cell communication between malaria-infected red blood cells via exosome-like vesicles. Cell 153:1120–1133
Schara K, Jansa V, Sustar V, Dolinar D, Pavlic JI, Lokar M, Kralj-Iglic V, Veranic P, Iglic A (2009) Mechanisms for the formation of membranous nanostructures in cell-to-cell communication. Cell Mol Biol Lett 14:636–656
Schorey JS, Cheng Y, Singh PP, Smith VL (2015) Exosomes and other extracellular vesicles in host-pathogen interactions. EMBO Rep 16(1):24–43
Silverman JM, Reiner NE (2011) Leishmania exosomes deliver preemptive strikes to create an environment permissive for early infection. Front Cell Infect Microbiol 1:26
Silverman JM, Clos J, Horakova E, Wang AY, Wiesgigl M, Kelly I, Lynn MA, McMaster WR, Foster LJ, Levings MK, Reiner NE (2010) Leishmania exosomes modulate innate and adaptive immune responses through effects on monocytes and dendritic cells. J Immunol 185:5011–5022
Tetta C, Ghigo E, Silengo L, Deregibus MC, Camussi G (2012) Extracellular vesicles as an emerging mechanism of cell-to-cell communication. Endocrine 44:11–19
Thery C, Ostrowski M, Segura E (2009) Membrane vesicles as conveyors of immune responses. Nat Rev Immunol 9:581–593
Torrecilhas AC, Schumacher RI, Alves MJ, Colli W (2012) Vesicles as carriers of virulence factors in parasitic protozoan diseases. Microbes Infect 14:1465–1474
Tovar J, Leon-Avila G, Sanchez LB, Sutak R, Tachezy J, van der Giezen M, Hernandez M, Muller M, Lucocq JM (2003) Mitochondrial remnant organelles of Giardia function in iron-sulphur protein maturation. Nature 426:172–176
Trocoli Torrecilhas AC, Tonelli RR, Pavanelli WR, da Silva JS, Schumacher RI, de Souza W, E Silva NC, de Almeida Abrahamsohn I, Colli W, Manso Alves MJ (2009) Trypanosoma cruzi: parasite shed vesicles increase heart parasitism and generate an intense inflammatory response. Microbes Infect 11:29–39
Twu O, de Miguel N, Lustig G, Stevens GC, Vashisht AA, Wohlschlegel JA, Johnson PJ (2013) Trichomonas vaginalis exosomes deliver cargo to host cells and mediate hostratioparasite interactions. PLoS Pathog 9:e1003482
Williams RL, Urbe S (2007) The emerging shape of the ESCRT machinery. Nat Rev Mol Cell Biol 8:355–368
Zhou Q, Sims PJ, Wiedmer T (1998) Identity of a conserved motif in phospholipid scramblase that is required for Ca2+-accelerated transbilayermovement of membrane phospholipids. Biochemistry 37(8):2356–2360
Acknowledgments
We would like to thank grants from Programa Parasitologia Basic (CAPES), CNPq, and Faperj. We also thank Instituto Oswaldo Cruz and Universidade Federal de Parana for hosting the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Evans-Osses, I., Reichembach, L.H. & Ramirez, M.I. Exosomes or microvesicles? Two kinds of extracellular vesicles with different routes to modify protozoan-host cell interaction. Parasitol Res 114, 3567–3575 (2015). https://doi.org/10.1007/s00436-015-4659-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-015-4659-9