Abstract
The risk of parasite infection grows with the size of host aggregations, which, in turn, may also depend on host sex and age and the quality of environmental resources. Herein, we studied the relationship between ectoparasitic infections with the wing mite (Spinturnix myoti) and the size of the breeding colonies, sex, age, and body condition index (BCI) of its host, the greater mouse-eared bat (Myotis myotis). The influence of environmental quality in the Carpathian Mountains (Poland) was also examined. We found significant differences in mite abundance and BCI between different breeding aggregations of the greater mouse-eared bat and also between the host sex/age categories. The most heavily infected bats were adult M. myotis females, while young males appeared to be the least infected. The BCI differed significantly between the sexes in young bats (males had a higher BCI than females) and also between colonies. No significant differences in the BCI were found for adult females. We did not find any relationship between the infestation rate of M. myotis, their colony size, the quality of environmental resources (percentage of forest cover around the colony), or the BCI. The prevalence of the various developmental stages of the mites did not differ between the host sex/age categories; however, differences were found in the sex ratios of deutonymphs and adult mites between adult M. myotis females. We predict that parasite load may not be dependent on colony size itself, but mainly on microclimatic factors, which are in turn directly correlated with colony size.
Similar content being viewed by others
Introduction
The risk of parasite infection increases with the size of animal aggregation (Combes 2001; Patterson and Ruckstuhl 2013). This pattern is particularly widespread among birds, which form large colonies, especially during the hatching and rearing periods (Brown and Brown 1986; Rózsa et al. 1996). A positive correlation between parasite infection intensity and host density has been observed in most colonial bird species (Brown and Brown 2000). This may be the result of an increased transfer of ectoparasites between hosts (mainly through horizontal transfer), which is indirectly confirmed by increased parasite infection in nonbreeding bird colonies (Blanco et al. 1997).
The level of infestation might be dependent not only on the number of individuals in aggregations but also on the density of hosts in the environment (Stanko et al. 2002). Parasite infections also depend on host sex; while in the majority of mammal species, males are more heavily infected than females (Krasnov et al. 2005; Morand et al. 2004), in bats, the preference for females is prevalent (Zahn and Rupp 2004; Christe et al. 2007; Patterson et al. 2008). Parasite preferences are determined by the condition of the host, which results from the availability of food resources (Khokhlova et al. 2002; Hawlena et al. 2006) and host physiology, and especially by immunosuppressive effects (Christe et al. 2000), which are in turn influenced by androgens (Klein 2004). Nevertheless, parasites can exploit both well-fed hosts in better condition, which are difficult to colonize, and vulnerable hosts in worse body condition, which are easier to colonize (Christe et al. 2003; Hawlena et al. 2005). Furthermore, ectoparasite reproduction is also strongly dependent on abiotic factors such as temperature and humidity, which can directly affect the level of parasite infection (Merino and Potti 1996; Hawlena et al. 2006; Pearce and O’Shea 2007).
Bats form some of the largest animal aggregations during both reproductive and nonreproductive seasons (winter aestivation) (Altringham 1999), which makes them a suitable model for the study of parasite–host relationships for colonial hosts. In two European species of bats, a positive correlation between the size of breeding colonies and the relative density of spinturnicid mites has been documented (Lučan 2006; Reckardt and Kerth 2009; Encarnação et al. 2012). In turn, no correlation between parasite infection and aggregation size has been found in two South Asian bat species, Tylonycteris sp. (Zhang et al. 2010). Higher ectoparasite infection rates in large host aggregations should have a negative impact on host condition, but the influence of ectoparasites on bat condition is still insufficiently understood. For example, significant differences in parasite load between bats (Myotis myotis) living in cave and attic colonies are not reflected in the condition of the host and its immune response (Uhrin et al. 2010).
In the present paper, we analyzed the parasitic parameters of the wing mite Spinturnix myoti infecting the greater mouse-eared bat M. myotis (Güttinger et al. 2001) in attic colonies in the Carpathian Mountains (southeastern Poland), differing with respect to the number of individuals. In the spring, adult M. myotis females form breeding colonies numbering from a few to hundred individuals (attics) or even several thousand individuals (caves). Colonies may be situated in attics (central Europe) or in caves (southern Europe) (Güttinger et al. 2001). At the end of May, females give birth to one young, which is nursed for about 3–4 weeks and becomes self-sufficient at 6 weeks of age (Pandurska 1998; Zahn 1999). Colonies disperse in mid-August (Zahn 1999; Zahn et al. 2006). The population density of M. myotis is strongly dependent on the percentage of forest cover around breeding colonies and is associated with the foraging habitat and availability of food (Zahn et al. 2006; Rudolph et al. 2009).
Spinturnicid mites are some of the most important hematophagous Acari (acarines) associated with European bats. These highly specialized ectoparasites live and reproduce exclusively on the wing or tail membranes of the host, and all their developmental stages (protonymphs, deutonymphs, and adult mites) feed on blood that can be stored in the midgut and its branches, including the mite’s legs (Evans 1968). Their life cycle is completely synchronized with the reproductive cycle of their hosts. In consequence, most European bat species carry the highest loads of spinturnicid mites during the bat breeding season (Zahn and Rupp 2004; Lourenço and Palmeirim 2008).
The aim of the present study was to investigate whether the infestation rate and the sex and age of the wing mite S. myoti are dependent on the sex, age, condition, environmental quality, and colony size of its host, M. myotis.
Materials and methods
Study sites
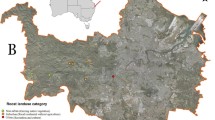
The study was conducted at the end of July in an area of 100 × 40 km located on the northern slopes of the Outer Carpathians (Beskids, Poland). In this region, woodless areas, coniferous forests, and deciduous forests form a mosaic. Meadows and farmlands are dominant in the lowlands, while at higher elevations, there is an increased proportion of deciduous and coniferous forests, with a minor presence of open areas (meadows). There are several dozens of known attic-breeding colonies of M. myotis in the Beskids (Kozakiewicz 2003; Szkudlarek et al. 2008; own data) (Fig. 1). The number of bats in breeding colonies ranges from 7 to 700 individuals (with an average of 145). Seven breeding colonies of M. myotis were selected which were at least several years old and differed in size from 50 to 250 individuals (females with young) (Table 1). All maternity aggregations were housed in church attics. For each of the seven colony sites, we calculated the percentage of forested area (potential foraging habitat) within a radius of 10 km around each nursery roost. The area of forest cover was assessed based on Google satellite maps using GetArea12c in CorelDraw 11.
Distribution of greater mouse-eared bat (Myotis myotis) maternity aggregations (grey circles) and single founding individuals (black dots) in the Beskids (Carpathian Mountains, Poland). Data pooled from Kozakiewicz (2003), Szkudlarek et al. (2008), and our data. For the investigated maternity colonies, forested areas within a 10-km radius is shown
The host and mites
The size of maternity colonies of the greater mouse-eared bat was evaluated on the basis of photographs of animal clusters with an accuracy of ±10 individuals. Due to the skittishness of bats, photographs were taken immediately after entering attics. M. myotis individuals were captured by hand directly from aggregations. Bats were placed in separate bags to prevent contamination of mite samples.
Regarding to the age of bats, the adult and first-year bats were distinguished on the basis of a “chin spot” and the level of ossification of the epiphyseal joints in the finger bones (Kunz 1988). Female lactation was determined on the basis of a lack of hair around nipples. Forearm length was measured with digital calipers, and bats were weighed with a Pesola scale with an accuracy of 0.25 g. The body condition index (BCI)/body mass (in grams) divided by forearm length (in mm) was used as a measure of bat condition (Speakman and Racey 1986). In further analyses, the following sex/age categories of bats were used: lactating adult females (fad lact), first-year females (fjuv), and first-year males (mjuv). Due to the sparse presence of nonlactating adult females, they were excluded from further analyses.
S. myoti mites (Fig. 2) were collected from bat wing membranes using tweezers and then fixed in 96 % alcohol. In addition, the fur of each host was screened for 30 s. Due to their marginal presence, other ectoparasites (one Nycteribiid and a few fleas) were excluded from further analysis.
The sex and developmental stage (protonymph, deutonymph, adult) of S. myoti were determined according to identification keys and other descriptions using light microscopy (Dusbábek 1962; Stanyukovich 1997; Rudnick 1960; Evans 1968; Uchikawa et al. 1994). The collected mites were deposited in the collection of the Faculty of Biology, Adam Mickiewicz University in Poznań. Thus, the prevalence of the examined bats was 100 %; in further analyses, we used only one parasitological parameter: mean intensity (MI), that is, the average number of parasites per infected individual in the sample (Bush et al. 1997).
Statistical analysis
Differences in the host BCI and ectoparasite MI (calculated following the procedure of Bush et al. (1997)) between host sex/age groups and breeding colonies were tested using ANOVA with a post hoc test (Tukey–Kramer honestly significant difference (HSD) with unequal samples). For further analysis (ANOVA and regression), we used standard transformations to approach normality: natural log transformation ln(x + 1) for MI and arcsine square root transformation for percentage of forested area (potential foraging habitat). The chi-square test (goodness of fit) was used to assess differences in the prevalence of mite stages between host sex/age groups and to compare sex ratios in deutonymphs and adult mites. Multiple regression analysis was used to test for the effects of the size of maternity aggregation, percentage of forest cover around the colony, and the BCI (independent variables) on the abundance of S. myoti. The Statistica 7 package (StatSoft Inc., Tulsa, OK, USA) was used for all statistical calculations.
Results
Hosts
During the study, 143 individuals of the greater mouse-eared bat (M. myotis) were caught: 59 lactating adult females, 39 young females, and 45 young males (Table 1). In adult females, the BCI did not differ significantly between colonies (one-way ANOVA: F = 1.58, df = 6.56, p = 0.172). In young bats, the BCI differed significantly between colonies (two-way ANOVA: F = 2.87, df = 6.70, p = 0.015) and sexes (F = 7.04, df = 1.70, p = 0.0098), while the interaction between sex and colony was not significant (F = 0.385, df = 6.70, p = 0.886). Bats from the Nowosielce colony were characterized by a lower BCI than those from colonies in Małastów (post hoc HSD test: p = 0.010), and young males had a higher BCI than young females (post hoc: p = 0.016).
Mites
A total of 2,270 mites were collected from bats, all belonging to the S. myoti species. In individual colonies, the MI of ectoparasites ranged from 11.3 ± 3.20 (±SE) to 23.3 ± 3.16 in lactating adult females, from 10.6 ± 1.69 to 20.0 ± 2.00 in young females, and from 8.0 ± 2.42 to 20.4 ± 5.48 in young males (Table 1).
Differences in parasite load were significant both between colonies (two-way ANOVA: F = 2.79, df = 6, p = 0.0142) and sex/age groups (F = 4.41, df = 2, p = 0.0141), while the sex/age × colony interaction was not significant (F = 0.59, df = 12, p = 0.8503).
Bats from the Harklowa colony were less infested than those from the Skalnik colony (post hoc HSD test: p = 0.0412). Among the sex/age groups, young males were infected significantly less than adult females (p = 0.011), but the differences between them and young females were not significant (p = 0.082). There were no significant differences in parasite load between young and lactating females (p = 0.851).
Among the collected mites, 1,178 adults, 385 deutonymphs, and 707 protonymphs were identified. Adult specimens were the most numerous (48.7–55.1 %), while deutonymphs were the least numerous (15.1–20.4 %); the proportion of protonymphs varied from 29.8 to 32.9 % (Fig. 3). In terms of the prevalence of particular stages of mites, lactating adult females did not differ from young females (chi-square = 2.02, df = 2, p < 0.365) or from young males (chi-square = 1.21, df = 2, p < 0.547). Similarly, young males and females did not differ in this respect (chi-square = 0.814, df = 2, p < 0.666).
The deutonymph sex ratio was similar in young bats, both in females (m/f = 50.4/49.6, chi-square = 0.04, p = 0.841) and males (m/f = 58.9/41.1, chi-square = 3.24, p = 0.072), whereas in lactating adult females, female mites were overrepresented (m/f = 40.0/60.0, chi-square = 4.02, p = 0.0455). A similar pattern was found among the adult stages of mites—no significant differences in their sex ratios were identified in young females (m/f = 40.6/59.4, chi-square = 3.24, p = 0.072) or males (m/f = 47.5/52.5, chi-square = 0.36, p = 0.548), while again an overrepresentation of female mites was found in lactating adult bat females (m/f = 39.3/60.7, chi-square = 4.84, p = 0.028). Differences in the sex ratios of deutonymphs and adult mites were only found for adult M. myotis females.
Hosts vs. ectoparasites
The influence of maternity aggregation size, percentage of forest cover around the colony, and the BCI on parasite abundance was not observed for any sex/age group. These three factors account for 4.9 % of variance in adult females (multiple regression: r 2 = 0.049, df = 3.55, F = 0.935, p = 0.430), 1.7 % for juvenile females (r 2 = 0.017, df = 3.35, F = 0.197, p = 0.898), and 2.4 % for juvenile males (r 2 = 0.024, df = 3.41, F = 0.336, p = 0.799) (Table 2, Fig. 4). Almost all variance results from intrapopulation variability.
Relationship between the size of bat maternity aggregation (a), percentage of forest cover (b), body condition index (c), and parasite infection of the examined bats in the Carpathians Mountains (2007). Triangles indicate adult females (solid line), grey circles juvenile females (dotted line), and black circles juvenile males (dashed line)
Discussion
Our results revealed (a) differences in mite abundance and the BCI between greater mouse-eared bat breeding colonies and between host sex/age categories; (b) no relationship between parasite abundance and the size of host breeding aggregations, host condition, or the surrounding environment; (c) no differences in the prevalence of particular developmental stages of mites between host sex/age categories; and (d) differences in the sex ratios of deutonymphs and adult mites between adult M. myotis females.
In contrast to other mammals (Côté and Poulin 1995), in most temperate bat species, females are significantly more heavily infected by ectoparasites than males, which is an example of female-biased infestation (Zahn and Rupp 2004; Christe et al. 2007; Patterson et al. 2008). No sex-biased preferences (Patterson et al. 2008) or male-biased infestation were found only in some species (Zhang et al. 2010). In most cases, shortly after birth, offspring are randomly infested by mites (without a clear preference for host sex; Christe et al. 2000). Those sex-biased preferences remain constant until the autumn movements (Lučan 2006; Christe et al. 2007). Female-biased infestation rates may be the cost of female sociality in most bat species (Reckardt and Kerth 2009). However, our observations indicate that in greater mouse-eared bats (M. myotis), the difference in infestation between young females and males increases during the lactation period, which cannot be explained by differences in the grooming activity rate or variance in immunocompetence. This may indicate that mites prefer one host sex over the other, which may be caused by hormonal signals (Klein 2004, 2005). In turn, sex/age groups of the host did not differ in terms of the prevalence of mite developmental stages (protonymph, deutonymph, and adult stages), suggesting a lack of preference for a particular sex or age of the host. The only differences in the sex ratios of mites were found in adult M. myotis females in the form of a female overrepresentation in deutonymph and adult stages. This disproportion in the sex ratio may be due to selective mite removal or the effect of selective mite preferences for female adult hosts. Size differences between the sexes in Spinturnicidae species should affect susceptibility to grooming: individuals of the larger sex could be more easily removed from wings by the host. Because S. myoti females are larger than males, we would expect the inverse proportion, that is, an overrepresentation of males (the effect of the grooming mortality of the larger sex), which was not the case. Selective choice of host sex and age by the different mite developmental stages and sexes has not been the subject of research yet. To fully elucidate the differences in the proportion of mite sexes, it would be necessary to study the Spinturnicidae life cycle, employing both field observations and laboratory experiments.
It is known that in species forming large aggregations, the parasite infection rate increases with colony size (Rózsa et al. 1996; Brown and Brown 2000; Krasnov et al. 2002). This pattern has also been observed in nonbreeding bird colonies (Blanco et al. 1997). In most cases, group size was positively correlated with the prevalence and intensity of parasites, which were typically directly transmitted (Patterson and Ruckstuhl 2013). A positive correlation of bat maternity aggregation size with infestation rates was found in two species: Myotis daubentonii (Lučan 2006; Encarnação et al. 2012) and Myotis bechsteinii (Reckardt and Kerth 2009). These two Myotis species mostly used tree holes or bat boxes for maternity roosts: breeding colonies in this type of shelters consist of a maximum of 144 individuals for M. daubentonii (Encarnação et al. 2005) and up to 45 individuals for M. bechsteinii (Reckardt and Kerth 2006, 2009). First, this type of shelters is very compact, which in the case of high host density may limit grooming and facilitate parasite transfer (both vertical and horizontal). On the other hand, an increased ectoparasite abundance results not only from better survival but also from greater reproduction, which is in turn highly dependent on temperature and humidity (Marshall 1982; Moyer et al. 2002; Bartonička and Gaisler 2007). The major effect of humidity on the reproduction of wing mites is consistent with their significantly higher abundance in cave breeding colonies than in attic ones, despite the similar size of host maternity aggregations (Uhrin et al. 2010; our data). In Eptesicus fuscus, a higher abundance of wing mites was observed in wet years than in dry seasons (Pearce and O’Shea 2007). Second, the thermal conditions of forest roosts are relatively stable (woodpecker tree holes; Kerth et al. 2001) or modified by external conditions only to a small extent: from a few (woodcrete bat boxes; Kerth et al. 2001) to several degrees above the external temperature (wooden bat boxes) (Lourenço and Palmeirim 2004; Kerth et al. 2001). The presence of bats in a small space also affects the microclimate of the refuge: the humidity and mean temperature inside bat boxes have been reported to be positively correlated with the number of bats due to their respiration (Bartonička and Řehák 2007). In contrast to forest shelters (bat boxes or tree holes), the microclimate of attics (humidity and temperature) is strongly dependent on the external temperature and is independent of the presence of bats (Zahn 1999; Lourenço and Palmeirim 2004; Postawa and Gas 2009; Uhrin et al. 2010). Hence, the direct cause of increased ectoparasite loads is probably not the size of the bat cluster but the higher humidity in the shelter. The variability of microclimate in attics (particularly low humidity) should explain the lack of correlation between mite abundance and the size of the host aggregation in our study.
The size, number, and distribution of nursery colonies are affected mainly by habitat quality and food supply (Speakman et al. 1991; Encarnação et al. 2005; Zahn et al. 2006). High availability of food resources would enable animals to compensate for the costs of parasite defense by increased food intake (Khokhlova et al. 2002; Hawlena et al. 2006). In turn, population density is correlated with parasite infection, which is commonly found in rodents (Stanko et al. 2002) and almost unexplored in bats. Our study revealed differences in the BCI and parasite load between colonies and sexes for juvenile bats, but a correlation between the BCI and parasite infections was not found. High population density should influence intraspecific competition (Zahn et al. 2006; Encarnação and Dietz 2006), hence the lack of correlation with bat BCI and the quality of the environment. In addition, the BCI of M. myotis juveniles may not be directly related to the availability and quality of food (scarce food resources) after they become self-sufficient but can also be affected by seasonal factors related to the time of birth (Arlettaz et al. 2001) or, more probably, the temperature during the first weeks of life (Zahn 1999; Postawa and Gas 2009).
Ectoparasites can affect the fitness of the host (Marshall 1982). An increase in the abundance of Spinturnix psi in Miniopterus schreibersii was negatively correlated with host condition during the nursing period, but not during pregnancy, mating, or hibernation (Lourenço and Palmeirim 2007). A high abundance of wing mites in M. myotis and M. daubentonii deteriorated host condition in adult females during pregnancy and in juvenile bats during autumn movements (Zahn and Rupp 2004). In the M. daubentonii–Spinturnix andegavinus system, a negative correlation during the post-lactation period and autumn movements was found for juvenile bats, and, unexpectedly, a positive correlation during the post-lactation period for adult and subadult females (Lučan 2006). In turn, the wing mite Spinturnix bechsteini infecting M. bechsteinii showed a higher abundance on hosts in medium condition (Reckardt and Kerth 2009). As can be seen, in some cases, bat ectoparasites exhibit a well-fed host selection strategy, while in other cases a vulnerable host selection strategy (Christe et al. 2003), and their impact on host condition seems to depend on the annual cycle and age of the host.
On the other hand, high parasite numbers in weak bats did not appear to be the cause, but rather a symptom, of their poor condition (Zahn and Rupp 2004). In our study, we found no relationship between parasite infection levels and the BCI. The absence of obvious interactions between the parasite infestation rate and host condition might be due to nonlinear associations between parasite load and host body condition, which have been suggested by some authors (Lučan 2006; Reckardt and Kerth 2009). In field studies of parasite–host models, often only one ectoparasite species is investigated, while others may be ignored even if their prevalence is similar. This can hinder interpretation because it is not known which parasite pressure influences the health status of the host. Another reason may be the imperfection of the BCI. In fact, the BCI reflects the lipid content of the host’s body (Pearce et al. 2008) but does not provide information about the host’s immunological response to the presence of parasites. An increase in the abundance of blood-sucking parasites induces greater energy expenditure due to higher blood consumption and at the same time leads to an increase in the amount of food intake in the host, but only marginally affects the host’s body condition (Khokhlova et al. 2002). Blood-sucking ectoparasites may affect the host’s status not only directly through the depletion of resources but can also be vectors of pathogens. For instance, Bartonella spp. (Hornok et al. 2012) as well as Bartonella spp. and Rickettsia spp. have been detected in the S. myoti collected during the breeding period of M. myotis (Szubert-Kruszyńska et al. 2009). Moreover, a high prevalence of viruses (coronaviruses, astroviruses, adenoviruses) has been found in the feces from a breeding colony of M. myotis (Drexler et al. 2011).
In conclusion, although permanent ectoparasites, including Spinturnicidae, are considered to be more independent of external climatic factors than temporary ones (Christe et al. 2007), abiotic factors seem to significantly affect parasite load in the investigated bat colonies. Hence, while the size of maternity aggregations is not directly correlated with parasite load, it can directly affect the microclimate of bat shelters and, as a result, indirectly influence the parasite infection rate. Consequently, the relationship between parasite load and host condition still remains unresolved and requires further studies to be better understood.
References
Altringham J (1999) Bats: biology and behaviour. Oxford University Press, Oxford, pp 1–262
Arlettaz R, Christe P, Lugon A, Perrin N, Vogel P (2001) Food availability dictates the timing of parturition in insectivorous mouse-eared bats. Oikos 95:105–111. doi:10.1034/j.1600-0706.2001.950112.x
Bartonička T, Řehák Z (2007) Influence of the microclimate of bat boxes on their occupation by the soprano pipistrelle Pipistrellus pygmaeus: possible cause of roost switching. Acta Chiropt 9:517–526. doi:10.3161/1733-5329(2007)9[517:IOTMOB]2.0.CO;2
Bartonička T, Gaisler J (2007) Seasonal dynamics in the number of parasitic bugs (Heteroptera, Cimcidae): a possible cause of roost switching in bats (Chiroptera, Vespertilionidae). Parasitol Res 100:1323–1330. doi:10.1007/s00436-006-0414-6
Blanco G, Tell JL, Potti J (1997) Feather mites on group living red-billed choughs: a non-parasitic interaction? J Avian Biol 28:197–206
Brown CR, Brown MB (1986) Ectoparasites as a cost of coloniality in cliff swallows (Hirundo pyrrhonota). Ecology 67:1206–1218
Brown CR, Brown MB (2000) Heritable basis for choice of group size in a colonial bird. Proc Natl Acad Sci U S A 97:14825–14830. doi:10.1073/pnas.97.26.14825
Bush AO, Lafferty KD, Lotz JF, Shostak AC (1997) Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol 83:575–583
Christe P, Arlettaz R, Vogel P (2000) Variation in intensity of a parasitic mite (Spinturnix myoti) in relation to the reproductive cycle and immunocompetence of its bat host (Myotis myotis). Ecol Lett 3:207–212
Christe P, Giorgi MS, Vogel P, Arlettaz R (2003) Differential species-specific ectoparasitic mite intensities in two intimately coexisting bat species: resource-mediated host attractiveness or parasite specialization? J Anim Ecol 72:866–872. doi:10.1046/j.1365-2656.2003.00759.x
Christe P, Glaizot O, Evanno G, Bruyndonckx N, Devevey G, Yannic G, Patthey P, Maeder A, Vogel P, Arlettaz R (2007) Host sex and ectoparasites choice: preference for, and higher survival on female hosts. J Anim Ecol 76:703–710. doi:10.1111/j.1365-2656.2007.01255.x
Combes C (2001) Parasitism. The ecology and evolution of intimate interactions. University of Chicago Press, Chicago, IL, USA
Côté IM, Poulin R (1995) Parasitism and group-size in social animals: a meta-analysis. Behav Ecol 6:159–165
Drexler JF, Corman VM, Wegner T, Tateno AF, Zerbinati RM, Gloza-Rausch F, Seebens A, Müller MA, Drosten C (2011) Amplification of emerging viruses in a bat colony. Emerg Infect Dis 17:449–456. doi:10.3201/eid1703.100526
Dusbábek F (1962) Parasitische Fledermausmilben der Tschechoslowakei I. Fam. Spinturnicdae Oudms., 1901 (Acarina, Gamasides). Časopis Československé Společnosti Entomologické. Acta Soc Ent Čechoslov 59(4):357–380
Encarnação JA, Kierdorf U, Holweg D, Jasnoch U, Wolters V (2005) Sex-related differences in roost-site selection by Daubenton’s bats Myotis daubentonii during the nursery period. Mamm Rev 35:285–294. doi:10.1111/j.1365-2907.2005.00066.x
Encarnação JA, Dietz M (2006) Estimation of food intake and ingested energy in Daubenton’s bats (Myotis daubentonii) during pregnancy and spermatogenesis. Eur J Wildl Res 52:221–227. doi:10.1007/s10344-006-0046-2
Encarnação JA, Baulechner D, Becker NI (2012) Seasonal variations of wing mite infestations in male Daubenton’s bats (Myotis daubentonii) in comparison to female and juvenile bats. Acta Chiropt 14:153–159. doi:10.3161/150811012X654367
Evans GO (1968) The external morphology of the post-embryonic developmental stages of Spinturnix myoti Kol. (Acari: Mesostigmata). Acarologia 10:589–608
Güttinger R, Zahn A, Krapp F, Schober W (2001) Myotis myotis (Borkhausen, 1797). Handb Säugetiere Eur Bd 4/I:123–207
Hawlena H, Abramsky Z, Krasnov BR (2005) Age-biased parasitism and density-dependent distribution of fleas (Siphonoptera) on a desert rodent. Oecologia 146:200–208. doi:10.1007/s00442-005-0187-0
Hawlena H, Krasnov BR, Abramsky Z, Khokhlova IS, Saltz D, Kam M, Tamir A, Degen AA (2006) Flea infestation and energy requirements of rodent hosts: are there general rules? Funct Ecol 20:1028–1036. doi:10.1111/j.1365-2435.2006.01190.x
Hornok S, Kovacs R, Meli ML, Gonczi E, Hofmann-Lehmann R, Kontschan J, Gyuranecz M, Dan A, Molnar V (2012) First detection of bartonellae in a broad range of bat ectoparasites. Vet Microbiol 159:541–543. doi:10.1016/j.vetmic.2012.04.003
Kerth G, Weissmann K, König B (2001) Day roost selection in female Bechstein’s bats (Myotis bechsteinii): a field experiment to determine the influence of roost temperature. Oecologia 126:1–9. doi:10.1007/s004420000489
Khokhlova IS, Krasnov BR, Kam M, Burdelova NI, Degen AA (2002) Energy cost of ectoparasitism: the flea Xenopsylla ramesis on the desert gerbil Gerbillus dasyurus. J Zool (Lond) 258:349–354. doi:10.1017/S0952836902001498
Klein S (2004) Hormonal and immunological mechanisms mediating sex differences in parasite infection. Parasite Immunol 26:247–264. doi:10.1111/j.0141-9838.2004.00710.x
Klein S (2005) Parasite manipulation of host behavior: mechanisms, ecology, and future directions. Behav Process 68:219–221. doi:10.1016/j.beproc.2004.07.009
Kozakiewicz K (2003) Letnie stanowiska nietoperzy na strychach budynków sakralnych w Beskidzie Niskim i Sądeckim oraz na Pogórzu Środkowobeskidzkim – kontrole 1999-2001. Stud Chiropterologica 3–4:21–30
Krasnov B, Khokhlova I, Shenbrot G (2002) The effect of host density on ectoparasite distribution: an example of a rodent parasitized by fleas. Ecology 83:164–175. doi:10.1890/0012-9658(2002)083[0164:TEOHDO]2.0.CO;2
Krasnov BR, Morand S, Hawlena H, Khokhlova IS, Shenbrot G (2005) Sex-biased parasitism, seasonality and sexual size dimorphism in desert rodents. Oecologia 146:209–217. doi:10.1007/s00442-005-0189-y
Kunz TH (1988) Ecological and behavioral methods for the study of bats. Smithsonian Institution Press, Washington, D.C, p 533
Lourenço S, Palmeirim J (2004) Influence of temperature in roost selection by Pipistrellus pygmaeus (Chiroptera): relevance for the design of bat boxes. Biol Conserv 119:237–243. doi:10.1016/j.biocon.2003.11.006
Lourenço S, Palmeirim J (2007) Can mite parasitism affect the condition of bat hosts? Implications for the social structure of colonial bats. J Zool 273:161–168. doi:10.1111/j.1469-7998.2007.00322.x
Lourenço S, Palmeirim J (2008) Which factors regulate the reproduction of ectoparasites of temperate-zone cave-dwelling bats? Parasitol Res 104:127–134. doi:10.1007/s00436-008-1170-6
Lučan R (2006) Relationships between the parasitic mite Spinturnix andegavinus (Acari: Spinturnicidae) and its bat host, Myotis daubentonii (Chiroptera: Vespertilionidae): seasonal, sex- and age-related variation in infestation and possible impact of the parasite on the host condition and roosting behaviour. Folia Parasitol 53:147–152
Marshall AG (1982) Ecology of insects ectoparasitic on bats. Plenum Press, New York and London, pp 369–397, w: ecology of bats. red.: Thomas Kunz
Merino S, Potti J (1996) Weather dependent effects of nest ectoparasites on their bird hosts. Ecography 19:107–113
Morand S, Gouy de Bellocq J, Stanko M, Miklisova D (2004) Is sex-biased ectoparasitism related to sexual size dimorphism in small mammals of Central Europe? Parasitology 129:505–510. doi:10.1017/S0031182004005840
Moyer BR, Drown DM, Clayton DH (2002) Low humidity reduces ectoparasite pressure: implications for host life history evolution. Oikos 97:223–228. doi:10.1034/j.1600-0706.2002.970208.x
Pandurska R (1998) Reproductive behaviour and conservation status of nursery colonies of Myotis myotis (Borkhausen, 1797) in Bulgaria. Myotis 36:143–150
Patterson BD, Dick CW, Dittmar K (2008) Parasitism by bat flies (Diptera: Streblidae) on neotropical bats: effects of host body size, distribution, and abundance. Parasitol Res 103:1091–1100. doi:10.1007/s00436-008-1097-y
Patterson JEH, Ruckstuhl KE (2013) Parasite infection and host group size: a meta-analytical review. Parasitology 140:803–813. doi:10.1017/S0031182012002259
Pearce RD, O’Shea TJ (2007) Ectoparasites in an urban population of big brown bats (Eptesicus fuscus) in Colorado. J Parasitol 93:518–530. doi:10.1645/GE-973R.1
Pearce RD, O’Shea TJ, Wunder BA (2008) Evaluation of morphological indices and total body electrical conductivity to assess body composition in big brown bats. Acta Chiropr 10:153–159. doi:10.3161/150811008X331171
Postawa T, Gas A (2009) Do the thermal conditions in maternity colony roost determine the size of young bats? Comparison of attic and cave colonies of Myotis myotis in Southern Poland. Folia Zool 58:396–408
Reckardt K, Kerth G (2006) The reproductive success of the parasitic bat fly Basilia nana (Diptera: Nycteribiidae) is affected by the low roost fidelity of its host, the Bechstein’s bat (Myotis bechsteinii). Parasitol Res 98:237–243. doi:10.1007/s00436-005-0051-5
Reckardt K, Kerth G (2009) Does the mode of transmission between hosts affect the host choice strategies of parasites? Implications from a field study on bat fly and wing mite infestation of Bechstein’s bats. Oikos 118:183–190. doi:10.1111/j.1600-0706.2008.16950.x
Rózsa L, Rekasi J, Reiczigel J (1996) Relationship of host coloniality to the population ecology of avian lice (Insecta: Phithiraptera). J Anim Ecol 65:242–248
Rudnick A (1960) A revision of the mites of the family Spinturnicidae, Acarina. Univ Calif Publ Entomol 17:157–284
Rudolph B-U, Liegl A, von Helversen O (2009) Habitat selection and activity patterns in the greater mouse-eared bat Myotis myotis. Acta Chiropt 11:351–361. doi:10.3161/150811009X485585
Speakman JR, Racey PA (1986) The influence of body condition on sexual development of male brown long-eared bats (Plecotus auritus) in the wild. J Zool (Lond) 210:515–525
Speakman JR, Racey PA, Catto CMC, Webb PI, Swift SM, Burnett AM (1991) Minimum summer populations and densities of bats in N.E. Scotland, near the northern borders of their distribution. J Zool (Lond) 225:32–345
Stanko M, Miklisová D, Gouy de Bellocq J, Morand S (2002) Mammal density and patterns of ectoparasite species richness and abundance. Oecologia 131:289–295. doi:10.1007/s00442-002-0889-5
Stanyukovich M (1997) Keys to gamasid mites (Acari, Parasitiformes, Mesostigmata, Macronyssoidea et Lelaptoidea) parasitizing bats (Mammalia, Chiroptera) from Russia and adjacent countries. Rudolstädter nat hist Schr 7:13–46
Szkudlarek R, Węgiel A, Węgiel J, Paszkiewicz R, Mleczek T, Szatkowski B (2008) Nietoperze Beskidu Sądeckiego i Niskiego. Nietoperze 9:19–58
Szubert-Kruszyńska A, Michalik J, Stańczak J, Cieniuch S, Podsiadły E (2009) Molecular survey of haematophagous spinturnicid mites (Acari: Spinturnicidae) and their bat host for rickettsial agents in Poland. X international Jena symposium on tick-borne diseases, Weimar, Germany, 19-21.03.2009, Abstract book, V 14, p. 38
Uchikawa K, Meng-Yu Z, O’Connor BM, Klompen H (1994) Contribution to the taxonomy of the genus Spinturnix (Acari: Spinturnicidae), with the erection of a new genus, Emballonuria. Folia Parasitol 41:287–304
Uhrin M, Kaňuch P, Krištofík J, Paule L (2010) Phenotypic plasticity in the greater mouse-eared bat in extremely different roost conditions. Acta Theriol 55:153–164. doi:10.4098/j.at.0001-7051.073.2009
Zahn A (1999) Reproductive success, colony size and roost temperature in attic-dwelling bat Myotis myotis. J Zoolo Lond 247:275–280
Zahn A, Rupp D (2004) Ectoparasite load in European vespertilionid bats. J Zool 262:383–391. doi:10.1017/S0952836903004722
Zahn A, Rottenwallner A, Guttinger R (2006) Population density of the greater mouse-eared bat (Myotis myotis), local diet composition and availability of foraging habitats. J Zool 269:486–493. doi:10.1111/j.1469-7998.2006.00081.x
Zhang L, Parsons S, Daszak P, Wei L, Zhu G, Zhang S (2010) Variation in the abundance of ectoparasitic mites of flat-headed bats. J Mammal 91:136–143. doi:10.1644/08-MAMM-A-306R2.1
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Postawa, T., Szubert-Kruszyńska, A. Is parasite load dependent on host aggregation size? The case of the greater mouse-eared bat Myotis myotis (Mammalia: Chiroptera) and its parasitic mite Spinturnix myoti (Acari: Gamasida). Parasitol Res 113, 1803–1811 (2014). https://doi.org/10.1007/s00436-014-3826-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-014-3826-8