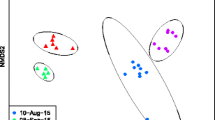
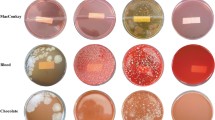
Abstract
Differences in midgut bacterial communities of Aedes aegypti, the primary mosquito vector of dengue viruses (DENV), might influence the susceptibility of these mosquitoes to infection by DENV. As a first step toward addressing this hypothesis, comparative analysis of bacterial communities from midguts of mosquito strains with differential genetic susceptibility to DENV was performed. 16S rRNA gene libraries and real-time PCR approaches were used to characterize midgut bacterial community composition and abundance in three Aedes aegypti strains: MOYO, MOYO-R, and MOYO-S. Although Pseudomonas spp.-related clones were predominant across all libraries, some interesting and potentially significant differences were found in midgut bacterial communities among the three strains. Pedobacter sp.- and Janthinobacterium sp.-related phylotypes were identified only in the MOYO-R strain libraries, while Bacillus sp. was detected only in the MOYO-S strain. Rahnella sp. was found in MOYO-R and MOYO strains libraries but was absent in MOYO-S libraries. Both 16S rRNA gene library and real-time PCR approaches confirmed the presence of Pedobacter sp. only in the MOYO-R strain. Further, real-time PCR-based quantification of 16S rRNA gene copies showed bacterial abundance in midguts of the MOYO-R strain mosquitoes to be at least 10–100-folds higher than in the MOYO-S and MOYO strain mosquitoes. Our study identified some putative bacteria with characteristic physiological properties that could affect the infectivity of dengue virus. This analysis represents the first report of comparisons of midgut bacterial communities with respect to refractoriness and susceptibility of Aedes aegypti mosquitoes to DENV and will guide future efforts to address the potential interactive role of midgut bacteria of Aedes aegypti mosquitoes in determining vectorial capacity for DENV.





Similar content being viewed by others
References
Ashelford KE, Chuzhanova NA, Fry JC, Jones AJ, Weightman AJ (2006) New screening software shows that most recent large 16S rRNA gene clone libraries contain chimeras. Appl Environ Microbiol 72:5734–5741
Beier MS, Pumpuni CB, Bizio JC, Davis JR (1994) Effect of paraaminobenzenoic acid, insulin and gentamicin on Plasmodium falciparum development in anopheline mosquitoes (Diptera: Culicidae). J Med Entomol 31:561–565
Campbell CL, Mummey DL, Schmidtmann ET, Wilson WC (2004) Culture-independent analysis of midgut microbiota in the arbovirus vector Culicoides sonorensis (Diptera: Ceratopogonidae). J Med Entomol 41:340–348
Chavshin AR, Oshaghi MA, Vatandoost H, Pourmand MR, Raeisi A, Enayati AA, Mardani N, Ghoorchian S (2012) Identification of bacterial microflora in the midgut of the larvae and adult of wild caught Anopheles stephensi: a step toward finding suitable paratransgenesis candidates. Acta Trop 121:129–134
Clemons A, Mori A, Haugen M, Severson D, Duman-Scheel M (2010) Aedes aegypti culturing and egg collection. Cold Spring Harb Protoc 2010:pdb.prot5507
Dessaux Y, Elmerich C, Faure D (2004) Violacein: a molecule of biological interest originating from the soilborne bacterium Chromobacterium violaceum. Rev Med Interne 25:659–662
Dinparast Djadid N, Jazayeri H, Raz A, Favia G, Ricci I, Zakeri S (2011) Identification of the midgut microbiota of An. stephensi and An. maculipennis for their application as a paratransgenic tool against malaria. PLoS One 6:e28484
Favia G, Ricci I, Damiani C, Raddadi N, Crotti E, Marzorati M, Rizzi A, Urso R, Brusetti L, Borin S, Mora D, Scuppa P, Pasqualini L, Clementi E, Genchi M, Corona S, Negri I, Grandi G, Alma A, Kramer L, Esposito F, Bandi C, Sacchi L, Daffonchio D (2007) Bacteria of the genus Asaia stably associate with Anopheles stephensi, an Asian malarial mosquito vector. Proc Natl Acad Sci USA 104:9047–9051
Felsenstein J (1989) PHYLIP—phylogeny inference package (version 3.2). Cladistics 5:164–166
Germi R, Crance JM, Garin D, Guimet J, Lortat-Jacob H, Ruigrok RW, Zarski JP, Drouet E (2002) Heparan sulfate-mediated binding of infectious dengue virus type 2 and yellow fever virus. Virology 292:162–168
Gonzalez-Ceron L, Santillan F, Rodriguez MH, Mendez D, Hernandez-Avila JE (2003) Bacteria in midguts of field-collected Anopheles albimanus block Plasmodium vivax sporogonic development. J Med Entomol 40:371–374
Good IJ (1953) The population frequencies of species and the estimation of population parameters. Biom 40:237–264
Gusmão DS, Santos AV, Marini DC, Bacci M Jr, Berbert-Molina MA, Lemos FJ (2010) Culture-dependent and culture-independent characterization of microorganisms associated with Aedes aegypti (Diptera: Culicidae) (L.) and dynamics of bacterial colonization in the midgut. Acta Trop 115:275–281
Halstead SB (1999) Is there an inapparent dengue explosion? Lancet 353:1100–1101
Herrera EM, Ming M, Ortega-Barria E, Pereira ME (1994) Mediation of Trypanosoma cruzi invasion by heparan sulfate receptors on host cells and penetrin counter-receptors on the trypanosomes. Mol Biochem Parasitol 65:73–83
Huber T, Faulkner G, Hugenholtz P (2004) Bellerophon: a program to detect chimeric sequences in multiple sequence alignments. Bioinformatics 20:2317–2319
Hugenholtz P, Goebel BM, Pace NR (1998) Impact of culture-independent studies on the emerging phylogenetic view of bacterial diversity. J Bacteriol 180:4765–4774
Ito J, Ghosh A, Moreira AL, Wimmer EA, Jacobs-Lorena M (2002) Transgenic anopheline mosquitoes impaired in transmission of a malaria parasite. Nature 417:452–455
Lane DJ, Pace B, Olsen GJ, Stahl DA, Sogin ML, Pace NR (1985) Rapid determination of 16S ribosomal RNA sequences for phylogenetic analyses. Proc Natl Acad Sci USA 82:6955–6959
Lohse DL, Linhardt RJ (1992) Purification and characterization of heparin lyases from Flavobacterium heparinurn. J Biol Chem 267:24347–24355
Love DC, Esko JD, Moser DM (1993) A heparin-binding activity on Leishmania amastigotes which mediates adhesion to cellular proteoglycans. J Cell Biol 123:759–766
Maidak BL, Cole JR, Parker CT Jr, Garrity GM, Larsen N, Li B, Lilburn TG, McCaughey MJ, Olsen GJ, Overbeek R, Pramanik S, Schmidt TM, Tiedje JM, Woese CR (1999) A new version of the RDP (Ribosomal Database Project). Nucleic Acids Res 27:171–173
Michel K, Kafatos FC (2005) Mosquito immunity against Plasmodium. Insect Biochem Mol Biol 35:677–689
Morlais I, Severson DW (2001) Identification of a polymorphic mucin-like gene expressed in the midgut of the mosquito, Aedes aegypti, using an integrated bulked segregant and differential display analysis. Genetics 158:1125–1135
Morlais I, Mori A, Schneider JR, Severson DW (2003) Targeted approach toward identification of candidate genes determining Plasmodium gallinceum susceptibility in Aedes aegypti. Mol Gen Genomics 269:753–764
Mourya DT, Gokhale MD, Pidiyar VJ, Barde PV, Patole MS, Mishra AC, Shouche YS (2002a) Study of the effect of the midgut bacterial flora of Culex quinquefasciatus on the susceptibility of mosquitoes to Japanese encephalitis virus. Acta Virol 46:257–260
Mourya DT, Pidiyar VJ, Patole MS, Gokhale MD, Shouche YS (2002b) Effect of midgut bacterial flora of Aedes aegypti on the susceptibility of mosquitoes to dengue viruses. Dengue Bull 26:190–194
Nadkarni MA, Martin FE, Jacques NA, Hunter N (2002) Determination of bacterial load by real-time PCR using a broad-range (universal) probe and primers set. Microbiology 148:257–266
Pidiyar VJ, Jangid K, Patole MS, Shouche YS (2004) Studies on cultured and uncultured microbiota of wild Culex quinquefasciatus mosquito midgut based on 16S ribosomal RNA gene analysis. Am J Trop Med Hyg 70:597–603
Pinzon-Ortiz C, Friedman J, Esko J, Sinnis P (2001) The binding of the circumsporozoite protein to cell surface heparan sulfate proteoglycans is required for Plasmodium sporozoite attachment to target cells. J Biol Chem 27:26784–26791
Polz MF, Cavanaugh CM (1998) Bias in template-to-product ratios in multitemplate PCR. Appl Environ Microbiol 64:3724–3730
Pumpuni CB, Beier MS, Nataro JP, Guers LD, Davis JR (1993) Plasmodium falciparum: inhibition of sporogonic development in Anopheles stephensi by Gram-negative bacteria. Exp Parasitol 77:195–199
Pumpuni CB, Demaio J, Kent M, Davis JR, Beier JC (1996) Bacterial population dynamics in three anopheline species: the impact on Plasmodium sporogonic development. Am J Trop Med Hyg 54:214–218
Rani A, Sharma A, Rajagopal R, Adak T, Bhatnagar RK (2009) Bacterial diversity analysis of larvae and adult midgut microflora using culture-dependent and culture-independent methods in lab-reared and field-collected Anopheles stephensi—an Asian malarial vector. BMC Microbiol 9:96–118
Ratcliffe NA, Whitten MMA (2004) Vector immunity in microbe–vector interactions. In: Gillespie SH, Smith GL, Osbourn A (eds) SGM symposium 63: microbe–vector interactions in vector-borne diseases. Cambridge University press, Cambridge, pp 199–262
Riehle MA, Jacobs-Lorena M (2005) Using bacteria to express and display anti-parasite molecules in mosquitoes: current and future strategies. Insect Biochem Mol Biol 35:699–707
Riehle MA, Moreira CK, Lampe D, Lauzon C, Jacobs-Lorena M (2007) Using bacteria to express and display anti-Plasmodium molecules in the mosquito midgut. Int J Parasitol 37:595–603
Schlein Y, Polacheck I, Yuval B (1985) Mycoses, bacterial infections and antibacterial activity in sandflies (Psychodidae) and their possible role in the transmission of leishmaniasis. Parasitology 90:57–66
Schloss PD, Handelsman J (2005) Introducing DOTUR, a computer program for defining operational taxonomic units and estimating species richness. Appl Environ Microbiol 71:1501–1506
Schneider JR, Mori A, Romero-Severson J, Chadee DD, Severson DW (2007) Investigations of dengue-2 susceptibility and body size among Aedes aegypti populations. Med Vet Entomol 21:370–376
Severson DW (1997) RFLP analysis of insect genomes. In: Crampton JM, Beard CB, Louis C (eds) The molecular biology of insect disease vectors. Chapman & Hall, London, pp 309–320
Sinnis P, Coppi A, Toida T, Toyoda H, Kinoshita-Toyoda A, Xie J, Kemp MM, Linhardt RJ (2007) Mosquito heparan sulfate and its potential role in malaria infection and transmission. J Biol Chem 282:25376–25384
Suzuki M, Giovannoni SJ (1996) Bias caused by template annealing in the amplification mixtures of 16S rRNA genes by PCR. Appl Environ Microbiol 62:625–630
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Terenius O, Lindh JM, Eriksson-Gonzales K, Bussière L, Laugen AT, Bergquist H, Titanji K, Faye I (2012) Midgut bacterial dynamics in Aedes aegypti. FEMS Microbiol Ecol 80:556–565
Thathy V, Severson DW, Christensen BM (1994) Reinterpretation of genetics of susceptibility of Aedes aegypti to Plasmodium gallinaceum. J Parasitol 80:705–712
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Tian X, Cheng X, Mao Z, Chen G, Yang J, Xie B (2011) Composition of bacterial communities associated with a plant–parasitic nematode Bursaphelenchus mucronatus. Curr Microbiol 62:117–125
Wang BJ, Liu Y, Jiang JT, Liu B, Liu SJ (2007) Microbial diversity in scorpion intestine (Buthus martensii Karsch). Wei Sheng Wu Xue Bao 47:888–893
Wang S, Ghosh AK, Bongio N, Stebbings KA, Lampe DJ, Jacobs-Lorena M (2012) Fighting malaria with engineered symbiotic bacteria from vector mosquitoes. Proc Natl Acad Sci USA 109:12734–12739
Wani AA, Suraksai VP, Siddharth J, Raghavan RG, Patole MS, Ranade D, Shouche YS (2006) Molecular analysis of microbial diversity associated with the Loanar soda lake in India: an impact crater in basalt area. Res Microbiol 157:928–937
Weisburg WG, Barns SM, Pelletier DA, Lane DJ (1991) 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol 173:697–703
Welburn SC, Maudlin I (1999) Tsetse–trypanosome interactions: rites of passage. Parasitol Today 15:399–403
WHO—World Health Organization (2009) Dengue and dengue haemorrhagic fever. Fact sheet no. 117. http://www.who.int/mediacentre/factsheets/fs117/en/index.html
World Health Organisation (1997) Dengue hemorrhagic fever: diagnosis, treatment, prevention and control, 2nd edn. WHO, Geneva
Xi Z, Ramirez JL, Dimopoulos G (2008) The Aedes aegypti toll pathway controls dengue virus infection. PLoS Pathog 4(7):e1000098
Xia X, Xie Z (2001) DAMBE: software package for data analysis in molecular biology and evolution. J Hered 92:371–373
Yan G, Severson DW (2003) Dynamics of molecular markers linked to the resistance loci in a mosquito-Plasmodium system. Genetics 164:511–519
Zahner V, Lucarotti CJ, McIntosh D (2008) Application of 16S rDNA-DGGE and plate culture to characterization of bacterial communities associated with the sawfly, Acantholyda erythrocephala (Hymenoptera, Pamphiliidae). Curr Microbiol 57:564–569
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
Neighbor-joining tree constructed from sequences affiliated to Pseudomonas sp. from 16S rRNA gene libraries of MOYO-R, MOYO-S, and MOYO strains. The tree was generated by using the neighbor joining method with Kimura 2 parameter distances in MEGA 4.0 software. Numbers at nodes indicate percent bootstrap values above 50 (1,000 replicates). The bar indicates the Jukes–Cantor evolutionary distance. In the sequence codes, first letters R, S, and M indicate MOYO-R, MOYO-S, and MOYO strain, respectively. (DOC 74 kb)
Rights and permissions
About this article
Cite this article
Charan, S.S., Pawar, K.D., Severson, D.W. et al. Comparative analysis of midgut bacterial communities of Aedes aegypti mosquito strains varying in vector competence to dengue virus. Parasitol Res 112, 2627–2637 (2013). https://doi.org/10.1007/s00436-013-3428-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-013-3428-x