Abstract
The characterization of the cross-reactive and species-specific antigens of Neospora caninum and Toxoplasma gondii is important in the exploration to determine the common mechanisms of parasite–host interaction and to improve the serological diagnosis; it is also useful for the selection of the cross-reactive antigens that could be used in the development of vaccines or drugs for controlling the diseases caused by these two parasites. In this study, cross-reactive and species-specific antigens between N. caninum and T. gondii tachyzoites were comprehensively investigated using a proteomics approach with the application of two-dimensional gel electrophoresis, immunoblot analysis, matrix-assisted laser desorption/ionization–time-of-flight mass spectrometry (MALDI-TOF-MS), and MALDI-TOF/TOF-MS analysis. Immunoblotting and mass spectrometry analysis revealed that at least 42 individual protein spots of N. caninum were reacted with the anti-N. caninum serum, among which at least 18 protein spots were cross-reacted with the anti-T. gondii serum. Moreover, at least 31 protein spots of T. gondii were reacted with the anti-T. gondii serum, among which at least 19 protein spots were cross-reacted with the anti-N. caninum serum. Furthermore, some new specific proteins were also identified in the N. caninum protein profile by searching Toxoplasma sequences or sequences from other organisms. This study substantiates the usefulness of proteomics in the immunoscreening of the cross-reactive or species-specific antigens of both parasites. In addition, the present study showed that there was significant homology in the antigenic proteome profiles between the two parasites. These observations have implications for the design of multicomponent common vaccines against both parasite infections.
Similar content being viewed by others
Introduction
The apicomplexan parasites of Toxoplasma and Neospora are intestinal parasites that are collectively referred to as coccidian; both are obligate intracellular protozoan parasites that cause a wide range of diseases in different host species (Dubey 1999). Neospora caninum is an important pathogen known to cause abortion in cattle; in addition, it causes neuromuscular disease in dogs and other animals (Anderson et al. 1995; Dubey and Lindsay 1996; Dubey 1999). There is no good evidence that N. caninum can infect or cause disease in people (Graham et al. 1999). Toxoplasma gondii is an important pathogen in humans and animals; in particular, it is known to be responsible for congenital neurological defects in the fetus and opportunistic infections in immunocompromised individuals (Harkins et al. 1998). N. caninum and T. gondii are structurally, genetically, and immunologically closely related protozoan parasites in the phylum Apicomplexa (Ellis et al. 1994; Mugridge et al. 1999). Because these two organisms have nearly identical morphology and can cause similar pathology and disease, N. caninum has often been incorrectly identified as T. gondii (Dubey et al. 2002). In addition, both neosporosis and toxoplasmosis have been regarded as economically important diseases as both parasites cause disease during pregnancy that may result in fetal death or birth of live congenitally infected offspring. Therefore, there is an urgent need for new diagnostic tools as well as drugs and vaccines to control the diseases of neosporosis and toxoplasmosis, which are respectively caused by N. caninum and T. gondii.
Several studies have shown that there is antigenic cross-reactivity between N. caninum and T. gondii by using immunoblotting, enzyme-linked immunosorbent assay (ELISA), immunohistochemical test, inhibition ELISA, and the pyrrolidine dithiocarbamate (PDTC)-PCR assay (Paré et al. 1995; McAllister et al. 1996; Sundermann et al. 1997; Harkins et al. 1998; Nishikawa et al. 2002; Silva et al. 2007). Previously, Liao et al. (2005, 2006) identified three proteins—protein disulfide isomerase (PDI), heat-shock protein 70 (HSP70), and ribosomal protein 1 (RP1)—as cross-reactive antigens between N. caninum and T. gondii by using cross-reactive monoclonal antibodies, and the antibodies from all of them could inhibit invasion of both parasites. In our previous studies, we also identified apical membrane antigen 1 (AMA1) and ribosomal phosphoprotein P0 as cross-reactive antigens between the two parasites, and the antibodies from them could inhibit the invasion or growth of both parasites (Zhang et al. 2007a, b). These studies revealed that cross-reaction between both parasites is indeed present; however, only a few cross-reactive antigens have been identified. Therefore, for a better understanding of the relationship between the two parasites, detailed information about the cross-reactive antigens for the infection process in N. caninum and T. gondii is necessary.
Although the two parasites are known to be closely related, they have basically different biological, morphological, and molecular characteristics (Dubey et al. 2002), as well as different host specificity and different subsequent clinical outcomes (Innes et al. 2007). The application of the PDTC-PCR assay also demonstrated that N. caninum and T. gondii tachyzoites differ largely with regard to the functional involvement of proteases in the adhesion and invasion of host cells (Naguleswaran et al. 2003). Therefore, the identification of these cross-reactive antigens would be useful to identify species-specific antigens from both parasites and improve the serological diagnosis of both protozoan infections.
In current studies, two important large-scale activities that use bioinformatics are genomics and proteomics. Proteomics refers to the analysis of the complete set of proteins or proteome. Proteomics is a useful tool for obtaining a more complete understanding of the biology of parasites, and this technology will identify life cycle stage-specific proteins implicated in invasion, survival, virulence, disease pathogenesis, and transmission (Belli et al. 2005). Protein expression analysis using preliminary two-dimensional gel electrophoresis (2-DE)-based protein separation technologies combined with immunoblot analysis of antigenic proteins and then coupled with matrix-assisted laser desorption/ionization–time-of-flight mass spectrometry (MALDI-TOF MS) and bioinformatics analysis provides a means to identify parasite-specific proteins and antigenic proteins that represent potential vaccine candidates or targets for serodiagnosis (Klade 2002; Seliger and Kellner 2002). Moreover, the proteomics approach has greatly enhanced the possibilities for the mapping and characterization of protein populations (Cohen et al. 2002). Furthermore, the availability of a tenfold coverage of the sequence for the T. gondii genome (www.toxodb.org/ToxoDB.shtml), EST sequence data available for N. caninum (http://genome.wustl.edu/data/est.cgi), and that of other apicomplexan parasites, such as Plasmodium falciparum, Eimeria acervulina, and Eimeria tenella, has given rise to a preliminary analysis of global protein expression in both parasites (Belli et al. 2005). The proteomics approach has been found to be useful for analyzing the proteomes of some parasitic organisms, e.g., Plasmodium (Choumet et al. 2007), Toxoplasma (Kawase et al. 2007), Trichomonas (De Jesus et al. 2007), Leishmania (Brobey et al. 2006), and Trypanosoma (Foucher et al. 2006) species, including their development, evolution, and pathogenicity. The first proteomics analysis in N. caninum was published by Lee et al. (2003). Reports on the identification of new antigens to improve serodiagnosis and the definition of the molecular difference between N. caninum and T. gondii via the application of proteomics, as well as on the identification of a number of homologue proteins between N. caninum and T. gondii tachyzoites, such as HSP70, tubulin (α- and β-chains), PDI, and actin, as well as enolase, which were believed to be common antigens in both parasites, were also published by Lee et al. (2004, 2005). Therefore, the proteomics approach offers a powerful tool to identify the cross-reactive and species-specific antigens between the two parasites.
In this study, 2-DE and immunoblot analysis were carried out to evaluate the differences between N. caninum and T. gondii; MS and bioinformatics analyses were carried out to identify and characterize the cross-reactive and species-specific antigens between two parasites and to further evaluate whether the cross-reactive antigens could be used as common vaccines or drugs for controlling the diseases caused by the two parasites.
Materials and methods
Parasite culture and purification
N. caninum tachyzoites (Nc-1 strain) and T. gondii tachyzoites (RH strain) were maintained in African green monkey kidney (Vero) cells cultured in a minimum essential medium (Sigma, MO, USA) supplemented with 8% heat-inactivated fetal bovine serum and 50 μg/ml kanamycin at 37°C in a 5% CO2 air environment. For the purification of tachyzoites, parasites and host cell debris were washed in cold phosphate-buffered saline (PBS), and the final pellet was resuspended in cold PBS and syringed three times with a 27-gauge needle. The parasites were then filtered through a 5.0-μm pore filter (Millipore, USA), washed twice with 10 ml of PBS, and pelleted at 1,500 rpm for 10 min.
Chemicals and reagents
Dithioerythritol (DTT), IPG strips (Immobiline DryStrip, pH 3–10, 13 cm), an IPG buffer (pH 3–10), and ECL detection reagents were all purchased from GE Healthcare/Amersham Biosciences (UK). Trifluoroacetic acid (TFA) was obtained from Merck (Darmstadt, Germany). Urea and CHAPS were purchased from Sigma. Acetone, acetonitrile (ACN), and 2-propanol were purchased from Wako (Japan).
Preparation of parasite lysates
N. caninum and T. gondii tachyzoites were harvested and purified as described above. Parasites were then resuspended in PBS; parasite suspensions were adjusted to 1 × 108 tachyzoites/ml, treated with a protease inhibitor (Roche, Germany), disrupted three times by a freeze–thaw cycle (liquid nitrogen and a water bath at 37°C), and then sonicated (BRANSON, Japan) in an ice slurry. After centrifugation (2,000 rpm, 10 min, 4°C), supernatants were collected, the protein concentration of the lysates was determined with a bicinchoninic acid (BCA) protein assay kit (Pierce, USA), and soluble antigen aliquots were stored at −80°C until use.
For 2-DE samples, parasites were disrupted in a lysis buffer (20 mM Tris-base) including a protease inhibitor cocktail followed by repeated cycles of freezing–thawing, sonication, and centrifugation. The lysates were precipitated with cold acetone. The dried lysates were mixed with a lysis buffer consisting of 7 M urea, 2 M thiourea, 1% (w/v) DTT, 4% (w/v) CHAPS, 0.5% Triton X-100, and a 0.5% (v/v) IPG buffer (pH 3–10). After centrifugation (15,000 rpm, 10 min), the supernatant was collected and stored at −80°C.
Production of anti-T. gondii and anti-N. caninum tachyzoite sera
Six-week-old BALB/c mice (SLC, Japan) were immunized intraperitoneally with tachyzoite (1 × 108/mouse) lysates in an equal volume of Freund’s complete adjuvant (Sigma) for the first injection. Mice were immunized with tachyzoite (0.5 × 108/mouse) lysates in Freund’s incomplete adjuvant (Sigma) at 2 and 4 weeks post-primary injection. The sera were collected 2 weeks after the last immunization. The pre-infection sera were used as controls. These sera were stored at −80°C until use. All animal experiments were conducted according to the guidelines provided by the Association for Assessment and Acceleration of Laboratory Animal Care International.
Two-dimensional electrophoresis, immunoblotting, and protein identification
Isoelectric focusing (IEF) was performed using the N. caninum lysates (50 mg) and T. gondii lysates (50 mg) on IPG strips (Immobline Dry Strips, pH 3–10, 13 cm) with a cup loading instrument on an IPGphor system (Amersham Biosciences) and focused via the application of 32 kV hs. After IEF, the IPG strip was equilibrated in solution A (50 mM Tris–HCl, pH 8.8, 6 M urea, 2% SDS, 30% glycerol, 1% (w/v) DTT) and solution B (solution A without DTT but with 2.5% (w/v) iodoacetamide) for 15 min at room temperature and inserted into a 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gel; the gel was sealed with 1% agarose.
The protein spots were visualized with colloidal Coomassie Brilliant Blue (CBB) G-250 or transferred onto nitrocellulose membranes (Schleicher & Schuell, Dassel, Germany) for immunoblotting. For image analysis, gels were stained using the PlusOne Silver Staining kit (GE Healthcare). For immunoblotting studies, the immunoblotting was performed as described by Lee et al. (2006). Briefly, the membranes were blocked for 1 h with TBS (15 mM Tris–HCl, pH 8, 140 mM NaCl) containing 0.05% Tween 20 (TBS/T) and 5% skim milk. The membranes were incubated either with pooled anti-T. gondii serum or anti-N. caninum serum (n = 5) diluted to 1:1,000 in TBS/T containing 5% skim milk for 2 h at room temperature. Following incubation with horseradish peroxidase-conjugated goat anti-mouse IgG (BETHYL, USA) for 1 h, the reactions were detected by chemiluminescence using an ECL detection kit (Amersham Biosciences). For quantitative analysis, all of the immunoblot images were developed after 1-min exposure. The CBB-stained gel and immunoblotting films were scanned with a UMAX image scanner (Umax Technologies, USA). The obtained images were analyzed using Shimadzu software (Nonlinear Dynamics, Japan). The gel images were calibrated for molecular weight (MW) and isoelectric point (pI) using two-dimensional SDS-PAGE standards (Bio-Rad, USA).
Trypsic digestion for MS analysis
After image analysis, protein spots of interest were manually excised from CBB-stained 2-DE gels. Excised gels were washed with destaining solutions (50% (v/v) ACN in 25 mM ammonium bicarbonate (NH4HCO3)) for 15 min. The gel plugs were washed twice with 100 μl of 25 mM NH4HCO3 and 100 μl of ACN. The cysteine residues were reduced by 50 μl of 10 mM DTT/25 mM NH4HCO3 at 56°C for 45 min and alkylated by 50 μl of 55 mM iodoacetamide/25 mM NH4HCO3 at room temperature for 30 min. After dehydration of the gel spots with 100% ACN and vacuum drying, the proteins were digested in a gel with a 2-μl digestion buffer containing sequencing grade modified 10 mg/ml trypsin (Promega, USA). After 30 min, 5 μl of 25 mM NH4HCO3 was added to keep the gel pieces wet during tryptic cleavage at 37°C overnight. To extract the peptides, a 5-μl 2% TFA solution was added and the samples then sonicated for 10 min. Five microliters 50% CAN was added to 1% TFA and then sonicated for 10 min. The volume of separated liquid was reduced to 2 μl under vacuum, and the peptides were again dissolved in 10 μl 0.1% TFA. The peptides were purified with Eppendorf Perfect Pure C-18 Tip (Eppendorf, Germany) before MS. The peptide solution was then spotted onto a MALDI sample plate for mass analysis.
Protein identification by MALDI-TOF and MALDI-TOF/TOF mass spectrometry
The trypsin-digested peptide mixture of the protein spot was analyzed using Autoflex MALDI-TOF/TOF MS systems (Bruker Daltonics, Germany). The instrument was operated in the delayed extraction mode with a delay time of 140 ns. Spectra were obtained by the sum of 1,000 and 4,000 consecutive laser shots in the MS and MS/MS modes, respectively. For the MALDI-TOF MS analysis, protein identification by MS was performed as described by Lee et al. (2003). The monoisotopic peptide masses were selected in a range between 300 and 3,800 Da. The proteins were identified by mass spectrometry data (MS or MS/MS) using Bruker Biotools software equipped with the MASCOT (Matrix Science) search engine (http://www.matrixscience.com) and the National Center for Biotechnology Information protein sequence database (http://www.ncbi.nlm.nih.gov). Searches were also performed using the annotated genome sequence available at http://www.toxodb.org. The mass tolerance was set at 100 ppm, and one missed cleavage site for tryptic peptides was allowed. Carbamidomethyl cysteines as a fixed modification and methionine oxidation as a variable modification were considered in the search. Peptide masses were assumed to be monoisotopic. To confirm the reliability of the MALDI-TOF MS and database search identifications, we carried out peptide fragmentation using MALDI-TOF/TOF MS analysis on protein spots. Proteins that were identified using at least two matching peptides presenting high-quality MS/MS spectra (MS/MS ion score higher than 40) were retained; the MS/MS fragment tolerance was defined as 0.5 Da.
Identification of the recombinant proteins, SDS-PAGE, and Western blot analysis
The cloning, expression, and purification of the recombinant N. caninum surface antigen 1 (SAG1), PDI, dense granule antigen 7 (GRA7), and ribosomal phosphoprotein P0 (RPP0) were performed as previously described (Chahan et al. 2003; Liao et al. 2006; Huang et al. 2007; Zhang et al. 2007b). The concentration of proteins was determined with a BCA protein assay kit (Thermo Scientific, USA). The proteins were stored at −80°C until use.
To determine the cross-reactive antigens and species-specific antigens, these purified proteins were separated on 12% SDS-PAGE under reducing conditions as described by Liao et al. (2005). Western blot analysis was performed using anti-T. gondii serum or anti-N. caninum serum, respectively, as described by Zhang et al. (2007a).
Results
Protein profiling of N. caninum and T. gondii lysates and immunoblot analysis
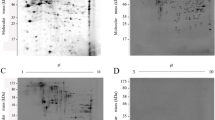
The proteome of the tachyzoite stage was profiled in N. caninum and T. gondii. By image analysis, most spots on 2-DE profiles and antigenic spots on 2-DE immunoblotting profiles were located at similar locations in terms of their isoelectric point and molecular weight, and all of the cross-reactive protein spots and species-specific protein spots were indicated in the N. caninum and T. gondii protein profiles according to the results of immunoblot analysis (Figs. 1, 2, and 3). Immunoblot analysis revealed that at least 42 protein spots of N. caninum were reacted with the anti-N. caninum serum (Fig. 3a), among which at least 18 protein spots were cross-reacted with the anti-T. gondii serum (Fig. 3b). Moreover, at least 31 protein spots of T. gondii were reacted with the anti-T. gondii serum (Fig. 3c), among which at least 19 spots were cross-reacted with the anti-N. caninum serum (Fig. 3d).
Tw-dimensional protein profiles of N. caninum. The lysates of N. caninum was separated by isoelectric focusing (pH 3–10) in the first dimension followed by SDS-PAGE in the second dimension on a 12% gradient gel. The antigenic spots corresponding to the specific proteins (blue numbers) and cross-reactive proteins (black numbers) are indicated
Two-dimensional protein profiles of T. gondii. The lysates of T. gondii was separated by isoelectric focusing (pH 3–10) in the first dimension followed by SDS-PAGE in the second dimension on a 12% gradient gel. The antigenic spots corresponding to the specific proteins (blue numbers) and cross-reactive proteins (black numbers) are indicated
Immunoblot analysis of the N. caninum and T. gondii antigenic spots. In the Western blot analysis of N. caninum tachyzoite lysates, the spots were probed with the N. caninum-specific serum (a) and the T. gondii-specific serum (b). In the Western blot analysis of T. gondii tachyzoite lysates, the spots were probed with the T. gondii-specific serum (c) and the N. caninum-specific serum (d). The cross-reactive antigenic spots are indicated by black numbers, and the specific antigenic spots are indicated by blue numbers. The serum was derived from mice immunized with tachyzoite lysates of the Nc-1 and RH strain
Identification of proteins as cross-reactive antigens by MALDI-TOF and MALDI-TOF/TOF MS
To understand the cross-reactive proteins, they were further identified using MALDI-TOF-MS and MALDI-TOF/TOF-MS. Protein spots were in-gel-digested and then analyzed via MALDI-TOF MS. Some protein spots corresponding to different protein entities were determined to have previously been registered in the GenBank genomic database. All of the proteins identified in N. caninum and T. gondii are summarized in detail in Table 1; a total of 73 protein spots from 2-DE were identified by MALDI-TOF MS and MALDI-TOF/TOF MS analysis. All of the proteins identified as cross-reactive antigens in N. caninum and T. gondii are listed in Table 2, which includes 18 cross-reactive spots in N. caninum and 19 cross-reactive spots in T. gondii determined by ECL (Fig. 3b, d). Database searching for protein identification was performed with the MASCOT search engine with mass spectrometry data (MS or MS/MS). The sequence alignment revealed that the proteins from N. caninum shared a significant degree of sequence identity with T. gondii. Among these cross-reactive proteins, major proteins were of the known immunostimulatory or immunogenic type, such as elongation factor-2, HSP90, and ribosomal phosphoprotein P0. A rhoptry protein is toxofilin. Some of the other cross-reactive proteins, including some enzymes from the energy metabolism, the phosphorylation pathway, the amino acid metabolism pathways, and diverse metabolic routes, which have also been reported as fructose-1,6-bisphosphate aldolase, enolase, protein disulfide isomerase, pyruvate kinase, actin, and lactate dihydrogenase, are believed to be highly conserved proteins between N. caninum and T. gondii.
Identification of proteins as species-specific antigens by MALDI-TOF and MALDI-TOF/TOF MS
The species-specific proteins of two parasites were identified using MALDI-TOF-MS and MALDI-TOF/TOF-MS, and these proteins are listed in Table 3, which includes nine species-specific proteins in T. gondii and 23 species-specific proteins in N. caninum. Database searching for protein identification revealed that some microneme, dense granule, and structural proteins were identified, and no host protein contamination in the proteome profile was detected. Some unidentified proteins are also listed in Table 3.
Identification of the recombinant proteins, SDS-PAGE, and Western blot analysis
For determination of the cross-reactive and species-specific antigens, four recombinant proteins were expressed in Echerichia coli as a GST fusion protein. The SDS analysis indicated that the recombinant N. caninum PDI-GST, RPP0-GST, GRA7-GST, SAG1-GST, and GST proteins had an approximate molecular weight of 76, 60, 57, 56, and 26 kDa, respectively (Fig. 4a), as calculated with Genetyx software (Genetyx Inc., Tokyo, Japan).
a SDS-PAGE analysis of the purified recombinant proteins. Purification of the recombinant proteins fused with GST was determined by 12% reducing SDS-PAGE. Purified recombinant proteins were subsequently used for Western blot analysis. Lane 1, PDI-GST; lane 2, RPP0-GST; lane 3, GRA7-GST; lane 4, SAG1-GST; lane 5, GST. b Western blot analysis of N. caninum PDI, RPP0, GRA7, SAG1, and GST proteins by mice anti-N. caninum serum. c Western blot analysis of N. caninum PDI, RPP0, GRA7, SAG1, and GST proteins by mice anti-T. gondii serum
Western blot analysis was performed with the mice anti-T. gondii serum or anti-N. caninum serum separately. The results revealed that these recombinant proteins of N. caninum reacted strongly with the mice anti-N. caninum serum, whereas the anti-T. gondii serum recognized the cross-reactive antigens PDI and RPP0, respectively. The purified GST protein was used as the control to react with the anti-T. gondii serum or anti-N. caninum serum, but no reacted bands were observed (Fig. 4b, c).
Discussion
There are only a few cross-reactive antigens that have been identified as important functional proteins that could inhibit the growth or invasion of both parasites so far (Liao et al. 2005, 2006; Zhang et al. 2007a, b). Therefore, it is difficult to find and characterize a good protein as a vaccine candidate to control the diseases caused by the two parasites. In the present study, the proteomics of a protein profile were proven to be highly useful for the identification of cross-reactive antigens between the two parasites; the approach described here allowed the identification of a large number of antigenic cross-reactive antigens. Moreover, the study methodology offered a better resolution to specifically identify common and distinct antigens between the two parasites than conventional SDS-PAGE protein separation. Furthermore, this method made it possible to identify antigenic cross-reactive proteins with a potential cross-protective effect and therefore provided some new candidate antigens for vaccine development to control both infections.
In the current study, two fascinating aspects of host–parasite relationships are how the two parasites interact with their hosts and how they cause diseases in different animal species. Based on these observations, we comprehensively immunoscreened the cross-reactive antigens used in the anti-serum against the two parasite lysates by proteomics; the protein spots on 2-DE profiles and antigenic spots on 2-DE immunoblotting profiles are shown in Figs. 1, 2, and 3. Among the identified cross-reactive proteins, HSP90 is a highly evolutionarily conserved protein and a potential drug target in T. gondii (Echeverria et al. 2005). Toxofilin is a rhoptry protein. It is likely to play a key role in the ability of the parasite to invade and co-opt the host cell for its own survival and growth (Bradley et al. 2005). Moreover, elongation factor-2 has been identified as a major immunostimulatory protein in Leishmania donovani and as a potential vaccine target against visceral leishmaniasis (Gupta et al. 2007). Another protein responsible for the virulence factor is an excretory–secretory product, PDI of N. caninum and T. gondii, which possesses PDI-specific enzymatic activity and could be a putative target for chemotherapy for neosporosis and toxoplasmosis (Meek et al. 2002; Liao et al. 2006). Some other proteins detected in MALDI analysis, such as glycolytic enzyme fructose-1,6-bisphosphate aldolase and enolase, may also be considered as potential vaccine candidates since they have been reported to be immunogenic in other organisms and have shown promise as good vaccine candidates, diagnostic tools, and drug targets (McCarthy et al. 2002; Pal-Bhowmick et al. 2004). In addition, in an investigation of the proteomes of virulent and attenuated BK strains of T. gondii tachyzoites by 2-DE coupled to MALDI-TOF-MS, NTPase I, catalase, and actin were identified to play a role in the virulence in this parasite (Nischik et al. 2001). As a consequence, despite the existent antigenic polymorphism between two parasites, the present study shows that there was significant homology in the proteome and antigenic proteome profiles between the two species; furthermore, these cross-reactive antigens could be potential vaccine candidates or targets for the control of infections by both parasites.
All in all, these findings also indicate that the two closely related species might not be all that similar. Comparisons of the two closely related parasites have revealed some interesting differences. In this study, some species-specific proteins were also characterized, including the excretory protein dense granule proteins, microneme proteins, heat shock proteins, glycolytic enzymes, and cytoskeletal proteins (Table 3). The species-specific proteins might be used as efficient serodiagnosis markers for the diagnosis of both parasites.
As shown in Fig. 3a–d, some spots exhibited a relatively high degree of antigenicity. These proteins represented major antigenic proteins. Some spots could not be identified. Unidentified proteins demonstrated relatively weak responses with antisera due to their low sequence coverage and limited availability of the N. caninum genomic database; however, no host proteins were detected. Therefore, we targeted these major protein spots for further analysis. In addition, cross-reactive antigens, which were obtained from the N. caninum protein profile, were slightly different from those obtained from the T. gondii protein profile. A probable explanation for these findings is the differential titers of antibodies to T. gondii and N. caninum in mouse sera or the differences in antibody affinity for the different antigens. Similar results were obtained in another study (Silva et al. 2007).
In this study, only the cross-reactive antigens of the tachyzoite stage of development were characterized by proteomics. However, analysis of the proteome of the bradyzoite/cyst stage of the parasite will reveal pathways for the conversion of tachyzoites to bradyzoites, and analysis of the cross-reactive antigens in the bradyzoite/cyst stage between two parasites will help identify novel proteins for the design of vaccines and drugs to treat chronic toxoplasmosis and neosporosis. Although the identification of the proteomes of the oocyst and cyst stages of development might be more challenging, they are being investigated in more detail for future studies.
In conclusion, this study demonstrates that the existence of cross-reactive antigens in both N. caninum and T. gondii and some cross-reactive antigens of N. caninum shared high homology with the corresponding antigens of T. gondii. Therefore, these cross-reactive antigens suggest that the host cell recognition and invasion in both species is similar. Moreover, comparative studies of the two closely related parasites have revealed some interesting differences. Consequently, further evaluation of the usefulness of the cross-reactive antigens as potential common vaccine candidates against both neosporosis and toxoplasmosis and the availability of the species-specific antigens as serological diagnosis candidates will be conducted in more detail in future studies.
References
Anderson ML, Palmer CW, Thurmond MC, Picanso JP, Blanchard PC, Breitmeyer RE, Layton AW, McAllister M, Daft B, Kinde H, Read DH, Dubey JP, Conrad PA, Barr BC (1995) Evaluation of abortions in cattle attributable to neosporosis in selected dairy herds in California. J Am Vet Med Assoc 207:1206–1210
Belli SI, Walker RA, Flowers SA (2005) Global protein expression analysis in apicomplexan parasites: current status. Proteomics 5:918–924
Bradley PJ, Ward C, Cheng SJ, Alexander DL, Coller S, Coombs GH, Dunn JD, Ferguson DJ, Sanderson SJ, Wastling JM, Boothroyd JC (2005) Proteomic analysis of rhoptry organelles reveals many novel constituents for host–parasite interactions in Toxoplasma gondii. J Biol Chem 280:34245–34258
Brobey RK, Mei FC, Cheng X, Soong L (2006) Comparative two-dimensional gel electrophoresis maps for promastigotes of Leishmania amazonensis and Leishmania major. Braz J Infect Dis 10:1–6
Chahan B, Gaturaga I, Huang X, Liao M, Fukumoto S, Hirata H, Nishikawa Y, Suzuki H, Sugimoto C, Nagasawa H, Fujisaki K, Igarashi I, Mikami T, Xuan X (2003) Serodiagnosis of Neospora caninum infection in cattle by enzyme-linked immunosorbent assay with recombinant truncated NcSAG1. Vet Parasitol 118:177–185
Choumet V, Carmi-Leroy A, Laurent C, Lenormand P, Rousselle JC, Namane A, Roth C, Brey PT (2007) The salivary glands and saliva of Anopheles gambiae as an essential step in the Plasmodium life cycle: a global proteomic study. Proteomics 7:3384–3394
Cohen AM, Rumpel K, Coombs GH, Wastling JM (2002) Characterisation of global protein expression by two-dimensional electrophoresis and mass spectrometry: proteomics of Toxoplasma gondii. Int J Parasitol 32:39–51
De Jesus JB, Cuervo P, Junqueira M, Britto C, Silva-Filho FC, Sabóia-Vahia L, González LJ, Barbosa Domont G (2007) Application of two-dimensional electrophoresis and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry for proteomic analysis of the sexually transmitted parasite Trichomonas vaginalis. J Mass Spectrom 42:1463–1473
Dubey JP (1999) Recent advances in Neospora and neosporosis. Vet Parasitol 84:349–367
Dubey JP, Lindsay DS (1996) A review of Neospora caninum and neosporosis. Vet Parasitol 67:1–59
Dubey JP, Barr BC, Barta JR, Bjerkås I, Björkman C, Blagburn BL, Bowman DD, Buxton D, Ellis JT, Gottstein B, Hemphill A, Hill DE, Howe DK, Jenkins MC, Kobayashi Y, Koudela B, Marsh AE, Mattsson JG, McAllister MM, Modrý D, Omata Y, Sibley LD, Speer CA, Trees AJ, Uggla A, Upton SJ, Williams DJ, Lindsay DS (2002) Redescription of Neospora caninum and its differentiation from related coccidia. Int J Parasitol 32:929–946
Echeverria PC, Matrajt M, Harb OS, Zappia MP, Costas MA, Roos DS, Dubremetz JF, Angel SO (2005) Toxoplasma gondii Hsp90 is a potential drug target whose expression and subcellular localization are developmentally regulated. J Mol Biol 350:723–734
Ellis J, Luton K, Baverstock PR, Brindley PJ, Nimmo KA, Johnson AM (1994) The phylogeny of Neospora caninum. Mol Biochem Parasitol 64:303–311
Foucher AL, McIntosh A, Douce G, Wastling J, Tait A, Turner CM (2006) A proteomic analysis of arsenical drug resistance in Trypanosoma brucei. Proteomics 6:2726–2732
Graham DA, Calvert V, Whyte M, Marks J (1999) Absence of serological evidence for human Neospora caninum infection. Vet Rec 144:672–673
Gupta SK, Sisodia BS, Sinha S, Hajela K, Naik S, Shasany AK, Dube A (2007) Proteomic approach for identification and characterization of novel immunostimulatory proteins from soluble antigens of Leishmania donovani promastigotes. Proteomics 7:816–823
Harkins D, Clements DN, Maley S, Marks J, Wright S, Esteban I, Innes EA, Buxton D (1998) Western blot analysis of the IgG responses of ruminants infected with Neospora caninum and with Toxoplasma gondii. J Comp Pathol 119:45–55
Huang P, Liao M, Zhang H, Lee EG, Nishikawa Y, Xuan X (2007) Dense-granule protein NcGRA7, a new marker for the serodiagnosis of Neospora caninum infection in aborting cows. Clin Vaccine Immunol 14:1640–1643
Innes EA, Bartley PM, Maley SW, Wright SE, Buxton D (2007) Comparative host–parasite relationships in ovine toxoplasmosis and bovine neosporosis and strategies for vaccination. Vaccine 25:5495–5503
Kawase O, Nishikawa Y, Bannai H, Zhang H, Zhang G, Jin S, Lee EG, Xuan X (2007) Proteomic analysis of calcium-dependent secretion in Toxoplasma gondii. Proteomics 7:3718–3725
Klade CS (2002) Proteomics approaches towards antigen discovery and vaccine development. Curr Opin Mol Ther 4:216–223
Lee EG, Kim JH, Shin YS, Shin GW, Suh MD, Kim DY, Kim YH, Kim GS, Jung TS (2003) Establishment of a two-dimensional electrophoresis map for Neospora caninum tachyzoites by proteomics. Proteomics 3:2339–2350
Lee EG, Kim JH, Shin YS, Shin GW, Kim YH, Kim GS, Kim DY, Jung TS, Suh MD (2004) Two-dimensional gel electrophoresis and immunoblot analysis of Neospora caninum tachyzoites. J Vet Sci 5:139–145
Lee EG, Kim JH, Shin YS, Shin GW, Kim YR, Palaksha KJ, Kim DY, Yamane I, Kim YH, Kim GS, Suh MD, Jung TS (2005) Application of proteomics for comparison of proteome of Neospora caninum and Toxoplasma gondii tachyzoites. J Chromatogr B Analyt Technol Biomed Life Sci 815:305–314
Lee EG, Na BK, Bae YA, Kim SH, Je EY, Ju JW, Cho SH, Kim TS, Kang SY, Cho SY, Kong Y (2006) Identification of immunodominant excretory–secretory cysteine proteases of adult Paragonimus westermani by proteome analysis. Proteomics 6:1290–1300
Liao M, Xuan X, Huang X, Shirafuji H, Fukumoto S, Hirata H, Suzuki H, Fujisaki K (2005) Identification and characterization of cross-reactive antigens from Neospora caninum and Toxoplasma gondii. Parasitology 130:481–488
Liao M, Ma L, Bannai H, Lee EG, Xie Z, Tang X, Zhang H, Xuan X, Fujisaki K (2006) Identification of a protein disulfide isomerase of Neospora caninum in excretory–secretory products and its IgA binding and enzymatic activities. Vet Parasitol 139:47–56
McAllister MM, Parmley SF, Weiss LM, Welch VJ, McGuire AM (1996) An immunohistochemical method for detecting bradyzoite antigen (BAG5) in Toxoplasma gondii-infected tissues cross-reacts with a Neospora caninum bradyzoite antigen. J Parasitol 82:354–355
McCarthy JS, Wieseman M, Tropea J, Kaslow D, Abraham D, Lustigman S, Tuan R, Guderian RH, Nutman TB (2002) Onchocerca volvulus glycolytic enzyme fructose-1,6-bisphosphate aldolase as a target for a protective immune response in humans. Infect Immun 70:851–858
Meek B, Back JW, Klaren VN, Speijer D, Peek R (2002) Protein disulfide isomerase of Toxoplasma gondii is targeted by mucosal IgA antibodies in humans. FEBS Lett 522:104–108
Mugridge NB, Morrison DA, Heckeroth AR, Johnson AM, Tenter AM (1999) Phylogenetic analysis based on full-length large subunit ribosomal RNA gene sequence comparison reveals that Neospora caninum is more closely related to Hammondia heydorni than to Toxoplasma gondii. Int J Parasitol 29:1545–1556
Naguleswaran A, Müller N, Hemphill A (2003) Neospora caninum and Toxoplasma gondii: a novel adhesion/invasion assay reveals distinct differences in tachyzoite–host cell interactions. Exp Parasitol 104:149–158
Nischik N, Schade B, Dytnerska K, Długońska H, Reichmann G, Fischer HG (2001) Attenuation of mouse-virulent Toxoplasma gondii parasites is associated with a decrease in interleukin-12-inducing tachyzoite activity and reduced expression of actin, catalase and excretory proteins. Microbes Infect 3:689–699
Nishikawa Y, Claveria FG, Fujisaki K, Nagasawa H (2002) Studies on serological cross-reaction of Neospora caninum with Toxoplasma gondii and Hammondia heydorni. J Vet Med Sci 64:161–164
Pal-Bhowmick I, Sadagopan K, Vora HK, Sehgal A, Sharma S, Jarori GK (2004) Cloning, over-expression, purification and characterization of Plasmodium falciparum enolase. Eur J Biochem 271:4845–4854
Paré J, Hietala SK, Thurmond MC (1995) An enzyme-linked immunosorbent assay (ELISA) for serological diagnosis of Neospora sp. infection in cattle. J Vet Diagn Invest 7:352–359
Seliger B, Kellner R (2002) Design of proteome-based studies in combination with serology for the identification of biomarkers and novel targets. Proteomics 2:1641–1651
Silva DA, Lobato J, Mineo TW, Mineo JR (2007) Evaluation of serological tests for the diagnosis of Neospora caninum infection in dogs: optimization of cut off titers and inhibition studies of cross-reactivity with Toxoplasma gondii. Vet Parasitol 143:234–244
Sundermann CA, Estridge BH, Branton MS, Bridgman CR, Lindsay DS (1997) Immunohistochemical diagnosis of Toxoplasma gondii: potential for cross-reactivity with Neospora caninum. J Parasitol 83:440–443
Zhang H, Compaore MK, Lee EG, Liao M, Zhang G, Sugimoto C, Fujisaki K, Nishikawa Y, Xuan X (2007a) Apical membrane antigen 1 is a cross-reactive antigen between Neospora caninum and Toxoplasma gondii, and the anti-NcAMA1 antibody inhibits host cell invasion by both parasites. Mol Biochem Parasitol 151:205–212
Zhang H, Lee EG, Liao M, Compaore MK, Zhang G, Kawase O, Fujisaki K, Sugimoto C, Nishikawa Y, Xuan X (2007b) Identification of ribosomal phosphoprotein P0 of Neospora caninum as a potential common vaccine candidate for the control of both neosporosis and toxoplasmosis. Mol Biochem Parasitol 53:141–148
Acknowledgments
This research was supported by a grant from The 21st Century COE Program (A-1) and a Grant-in-Aid for Scientific Research, both from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, H., Lee, Eg., Yu, L. et al. Identification of the cross-reactive and species-specific antigens between Neospora caninum and Toxoplasma gondii tachyzoites by a proteomics approach. Parasitol Res 109, 899–911 (2011). https://doi.org/10.1007/s00436-011-2332-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-011-2332-5