Abstract
In parasites of the order Kinetoplastida, such as Trypanosoma cruzi and Trypanosoma brucei, glycolysis is carried out by glycolytic enzymes in glycosomes. One of the glycolytic enzymes is triosephosphate isomerase (TIM), which in T. brucei is localized exclusively in glycosomes, whereas in T. cruzi, the localization of TIM has not been fully ascertained. In the present work, we made a monoclonal antibody (mAb 6-11G) against recombinant T. cruzi TIM (rTcTIM). Incubation of T. cruzi epimastigotes with the mAb inhibited parasite survival. Western blotting showed that the mAb recognized rTcTIM and a 27 kDa band in T. cruzi lysates that corresponded to TcTIM. Sera from patients with Chagas disease recognized rTcTIM and cross-reacted with human recombinant TIM. The cross reactivity between parasite and human TIM possibly contributes to the autoimmune pathogenesis of Chagas disease. Electron microscopy of T. cruzi epimastigotes with the mAb showed that TIM was located within glycosomes, in the cytoplasm, the nucleus, and the kinetoplast. Collectively, the data shed new light on T. cruzi TIM and opens perspectives for drug design.
Similar content being viewed by others
Introduction
Trypanosoma cruzi causes American trypanosomiasis or Chagas disease, a major cause of morbidity and mortality in Latin America. Treatment and prevention remain unsatisfactory because chemotherapy is nonspecific and generally has toxic side effects; furthermore, no treatment exists for the chronic phase of the disease (Urbina et al. 1996). Nitrofuranes and nitroimidazoles are used in the acute phase, but their efficacy varies widely according to geographic zones, and their toxicity remains a problem (Nozaki et al. 1996). The antigenic variation expressed by the parasite in different stages of its life cycle has limited the progress in vaccine development (WHO 1991). Thus, the development of drugs targeted against unique and vital parasite enzymes is currently a field of intense research. Enzymes of the glycolytic pathway are potential targets, since Trypanosomes rely heavily on glycolysis as an energy source (Visser and Opperdoes 1980). In the glycolytic pathway of Trypanosomes and Leishmanias, there are nine enzymes that convert glucose into 3-phosphoglycerate and glycerol, and one of these enzymes is triosephosphate isomerase (TIM), which is required for parasite growth and survival (Opperdoes and Borst 1977). This enzyme is one of the potential enzymes that can be targeted for species-specific inhibition (Ostoa-Saloma et al. 1997). TIM, together with the other first six glycolytic enzymes, is contained in glycosomes, microbody-like organelles that are a distinctive feature of the order of Kinetoplastida (Opperdoes 1987; Opperdoes and Michels 1993; Hannaert and Michels 1994). This organelle has a single-unit membrane that forms a permeability barrier to the intermediates of glycolysis (Visser and Opperdoes 1980). The subcellular compartmentalization of glycolysis in Kinetoplastida is fundamentally different from all other eukaryotic organisms, where glycolysis is carried out in the cytoplasm. Glycosomes have been extensively studied in Trypanosoma brucei (Hannaert and Michels 1994), where they have an average diameter of 0.27 μm and contain an electron-dense matrix, resembling microbodies or peroxisomes from other eukaryotic cells. In T. cruzi, it has previously been shown that early glycolytic enzymes are contained in glycosomes, which seem to be similar to those described in T. brucei. Their size varies between 0.1 and 0.25 μm in fixed whole epimastigotes (Taylor et al. 1980, Taylor and Gutteridge 1987; Hart and Opperdoes 1984). Yet, it remains unknown if, in T. cruzi, TIM is located exclusively in their glycosomes, or if they also have an extra-glycosomal distribution. Preliminary subcellular fractionation studies of T. cruzi indicated that TIM could be found in both the glycosomal and the soluble fraction (Gómez-Puyou et al., unpublished results). The purpose of our study was to produce a monoclonal antibody (mAb) against recombinant TIM from T. cruzi (rTcTIM), and examine its effect on T. cruzi epimastigotes, as well as to analyze the ultrastructural localization of the enzyme. We found that the mAb exerted an inhibitory effect on parasite growth and revealed multiple distribution sites of TIM in T. cruzi epimastigotes. We also found that T. cruzi Tim is an immunogenic molecule that shares epitopes with human TIM.
Materials and methods
Antigen preparation
Recombinant T. cruzi TIM (rTcTIM) was prepared as previously described (Ostoa-Saloma et al. 1997). In brief, expression was induced in Escherichia coli cells strain BL23 (DE3) pLysS. Cells were then resuspended in 25 mM Mes, 1 mM ethylenediaminetetraacetic acid (EDTA), 1 mM dithiothreitol, and 0.2 mM phenylmethylsulfonyl fluoride, pH 6.5. After lysis and centrifugation, ammonium sulfate was added to the supernatant to 45% and thereafter to 75% saturation. The precipitate was dissolved in 25 mM triethanolamine, 2 mM EDTA, 1mM azide, and 0.5 mM dithiothreitol, pH 8.0 and applied to an AcA 34 A column. The eluted fractions with activity were applied to a Carboxymethyl–Sepharose column (Fast flow, Pharmacia, Uppsala, Sweden). The eluted fractions with high activity from this second column were analyzed in sodium dodecyl sulfate (SDS) gels, and the purified enzyme was stored at 4°C as a 70% ammonium sulfate suspension.
Monoclonal antibody production
Syngeneic 6- to 8-week-old female Bagg albino/c (BALB/c) mice were immunized intraperitoneally with 50 μg rTcTIM in Freund’s complete adjuvant. Approximately 1week later, they received one intraperitoneal booster injection with 50 μg rTcTIM in Freund’s incomplete adjuvant and on day16, a final intravenous injection of 50 μg rTcTIM without adjuvant. The mice were killed 4 days later, and their spleen cells were fused with X63 Ag 8.653 myeloma cells. Subsequent culture selection and cloning of fused cells were performed as previously described (Zimmermann et al. 1994). Selection of positive hybridomas was made by enzyme-linked immunosorbent assay (ELISA), adding 0.65 μg of rTcTIM in 50 μl coating buffer (100 mM Na2CO3, 100 mM NaHCO3, pH 9.6) per well overnight at 4°C. After being washed with phosphate-buffered saline (PBS), pH 7.4, the plate was blocked for 1 h at room temperature with 1.6% skim milk and 0.05% Tween 20 in PBS (blocking solution). Thereafter, 50 μl samples of the supernatants from different cloned hybridoma cultures were incubated in different wells at room temperature for 2 h. The plate was washed extensively with PBS and peroxidase labeled rabbit anti-mouse (H + L chains) antibody (Zymed, San Francisco, CA, USA) was added in blocking solution and incubated for an additional 2 h at room temperature. The plate was washed extensively, and the reaction was developed for 20 min at 37°C with 100 μl of the substrate 2,2′amino-bis(3-ethylbenz-thiazoline-6-sulfonic acid) (100 mM; Sigma Chemical, St Louis, MO, USA), 5 ml of 100 mM citric acid pH 4.2, and 5 μl of 30% H2O2 for 20 min. Optical density in the wells was determined in an ELISA reader at 405 nm with a reference filter of 490 nm. Five clones were obtained and one clone, denoted 6-11G, was used in all studies. The immunoglobulins were purified by adding saturated ammonium sulfate with culture supernatants of the hybridoma cells or with the ascitis fluid, and they were allowed to precipitate for 12 h at 4°C under constant slow stirring. After centrifugation at 28,000×g for 30 min (Sorvall RC 5C, Du Pont), the pellet was resuspended in one third of the original volume of 10 mM Tris–HCl, pH 7.4 and dialyzed extensively against the same solution. The dialyzed precipitate was then concentrated five to seven times the initial volume in an Amicon system (Beverly, MA, USA) with membranes that have a cut-off molecular weight of 10,000 kDa (Diaflo, Amicon USA) and thereafter purified using a protein G affinity column.
Growth of T. cruzi epimastigotes in the presence of the mAb 6-11G
The parasites (1 × 106) were cultured in 1ml Roswell Park Memorial Institute (RPMI) 1640 (Gibco, Grand Island, NY, USA) in the presence of 9.6 mg/ml protein of precipitated ascitis fluid of clone 6-11G during 8 days at 28°C. Parasite growth was monitored after 24, 48 and 72 h and every third day thereafter, until day 8, by parasite counts in a Neubauer chamber. As control, parasites were incubated in a nonrelated ascitis fluid or RPMI.
Western blot analysis
Epimastigotes (8 × 108) were lysed by three cycles of sonication of 3 min at 4°C and 37% intensity in 1ml lysis buffer containing 10 mM imidazole, pH 7.2, 5 mM EDTA, 0.1% β-mercaptoethanol and 2 μg/ml leupeptine, 10 μg/ml aprotinin, 1mM benzamidine, and 1% Triton X-100 as described previously (Aguirre-García et al. 2000). Parasite lysis was verified by microscopy. Twenty μg of whole parasite lysate proteins or rTcTIM were separated by SDS polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto Immobilon membranes. Membranes were blocked with 5% nonfat milk for 12 h, washed twice with 5% Tris-buffered saline Tween-20 (TBST). The blot was incubated with mAb 6-11G, diluted 1:100, for 3 h at 37°C and the secondary antibody goat anti-mouse alkaline phosphatase-labelled IgG, diluted 1:5,000, for 3 h at 4°C. Alkaline phosphatase was developed with 0.5 mg/ml 3′diaminobenzidine (Sigma Chemical) in 100 mM Tris–HCl, pH 7.4, containing 100 ml of 30% H2O2.
A pool, as well as four individual sera, from patients with Chagas disease was also used to analyze their recognition of rTcTIM. Sera from patients were diluted 1:50 and incubated with the blotted rTcTIM for 12 h at 4°C. In this case, the secondary antibody was goat-antihuman IgG (Zymed) in 1% bovine serine albumin (BSA) in TBST diluted 1:10,000 for 1 h at room temperature. Blots were developed for AP (Sambrook et al. 1989).
In addition, we analyzed by Western blot if the mAb 6-11G and sera from patients with Chagas disease also recognize human recombinant TIM. This was done using the same conditions as described for rTcTIM.
Fluorescent staining of epimastigotes of T. cruzi with mAb 6-11G
One million epimastigotes were washed twice with PBS and fixed in cold acetone for 20 min. The cells were washed twice in TBS (10 mM Tris–HCl, pH 7.4, 150 mM NaCl).
The epimastigotes were blocked with 1% PBS, 1% BSA, and 0.01% Triton-X-100 for 2 h and incubated with 1ml of 6-11G diluted 1:1,000 in PBS (0.1% BSA) or normal mouse IgG as a control for 3 h at room temperature with end-over-end agitation. They were washed twice with TBS; fluorescein isothiocyanate-labeled goat anti-mouse IgG antibody (Zymed) 1:1,000 was added and incubated for 2 h at room temperature with shaking. The cells were washed twice with TBS and observed under a microscope with epifluorescent illumination.
Post-embedding immunogold studies
Ultrastructural immunocytochemistry was performed on epimastigotes obtained from cultures. Epimastigotes were resuspended and fixed with 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer, pH 7.2, during 2 h at 4°C. After washing three times in the same buffer with 5% sucrose, parasites were pelleted at 1,500×g for 10 min and post-fixed in a solution of 1% OsO4 for 10 min at 4°C. After being washed, the parasites were dehydrated and embedded in LR White medium to preserve the antigenicity of TIM (Bozzola and Russell 1998). Ultrathin sections were mounted on nickel grids coated with Formvar and processed with an immunogold postembedding protocol. Briefly, grids were floated onto a drop of sterile PBS for 5 min at room temperature (RT) and then onto a drop of 2% bovine serum albumin (BSA) in PBS containing 0.01% Triton-X-100 for 1 h at RT for blocking nonspecific sites. Without rinsing, the grids were incubated with the primary mAb 6-11G diluted 1:1,000 in PBS (0.1% BSA) overnight at 4°C in a moist chamber. Then, grids were thoroughly washed with sterile PBS and incubated with goat anti-mouse IgG tagged with 10 or 20 nm gold particles (ICN, Costa Mesa, CA, USA) diluted 1:50 in PBS (0.1% BSA) for 1 h at room temperature in a moist chamber. Finally, the grids were thoroughly washed with PBS and deionized water and contrasted with 2% aqueous uranyl acetate. Ultrastructural analysis was carried out using a Zeiss EM-10 electron microscope. Controls were incubated with a nonrelated monoclonal antibody or normal mouse IgG diluted 1:1,000 as primary antibody.
Purification of glycosomes from T. brucei
T. brucei was purified from blood of infected rats following the method described (Lanham 1968). The purification of glycosomes was done according to the method previously described (Aman and Wang 1986; Opperdoes et al. 1984). In brief, bloodstream forms of T. brucei brucei were grown in Wistar rats. After 4 days, blood was collected by cardiac puncture in anesthesized animals which had high parasitemias. Trypanosomes were passed through a diethylamino ethanol–cellulose column to separate them from erythrocytes (Lanham 1968). Cells were washed several times with buffer containing 0.25 M sucrose, 25 mM Tris–HCl, 1 mM EDTA (pH 7.8), and centrifuged (1,000×g). The washed pellet was disrupted by grinding with silicon carbide in a buffer containing 0.25 M sucrose, 25 mM Tris–HCl, 1 mM EDTA (pH 7.4). After centrifugation (1,000 × g), the supernatant was mixed with an equal volume of 80% Percoll containing 0.25 M sucrose, 25 mM Tris–HCl, and 1mM EDTA, pH 7.2, to achieve a final Percoll concentration of 40%. It was centrifuged (59,000×g) for 30 min in a vertical rotor. The lower band enriched in glycosomes, equilibrating at 1.09 g/cm3, was removed after the side of the tube was punctured with a syringe. This suspension was layered on a linear 0.4–2.7 M sucrose gradient containing 25 mM Tris–HCl, pH 7.2, and 1 mM EDTA and centrifuged at 193,000 × g for 90 min in a vertical rotor. The highly purified glycosomes, which equilibrated at a density of 1.23 g/cm3, were obtained by a syringe after puncturing the side of the tube.
Electron microscopy studies of TIM in purified T. brucei glycosomes
The electron microscopy analysis was carried out following the same procedure described above with minor modifications. Organelles were fixed in the same fixative but containing 250 mM sucrose (pH 7.5), for 1 h at 4°C; then they were pelleted in a microfuge (10,600 × g for 10 min) and washed twice in sodium cacodylate buffer and postfixed in 1% OsO4 for 5 min at 4°C. Thereafter, they were dehydrated and embedded in Epon 812. Ultrathin sections mounted on nickel grids were processed for immunogold procedure in the same way as described for complete parasites.
Results
Antibody production
The fusion of splenocytes of mice immunized with rTcTIM with the myeloma cells produced a hybridoma clone capable of secreting a specific mAb (6-11G) against TcTIM. This was detected by ELISA tests. Additionally, we found that 6-11G also recognized recombinant TIM from T. brucei (rTbTIM) (data not shown).
In vitro growth inhibition
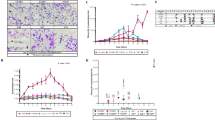
When T. cruzi epimastigotes were cultured in vitro in the presence of 9.6 mg/ml of mAb 6-11G for 8 days, a marked loss of viability was observed after the first 24 h of coculture. After 5 days, no living epimastigotes could be detected (Fig. 1). In contrast, no growth inhibition was observed when a non-T. cruzi related control monoclonal antibody (W6) was used; instead, it exerted a stimulatory effect on the growth of the epimastigotes.
Western blot
To analyze the specificity of the mAb, Western blots were done using whole parasite lysates as well as rTcTIM as antigens. T. cruzi lysate proteins as well as rTcTIM were separated in SDS-PAGE and transferred onto immobilon-P membranes for Western blotting (Fig. 2). In the Coomassie stained gel (Fig. 2a), proteins of the parasite lysate are shown in lane 2; rTcTIM protein was placed in lane 3, it exhibited a single 27 kDa band. The Western blot analysis of the T. cruzi lysate shows that the mAb against rTcTIM only recognizes a band of 27 kDa (lane 4). The Western blot analysis of the rTcTIM shows that the same 27 kDa band is recognized by the mAb (lane 5). Nonrelated mAbs or the use of secondary anti-mouse IgG did not show any reactivity with rTcTIM (data not shown). These results indicate that the mAb against rTcTIM is specific for TIM, since it does not recognize any other band present in the parasite lysate. Additionally, we tested if a pool of sera from four patients with acute Chagas disease could recognize rTcTIM. A band with a molecular mass of 27 kDa with intense staining was observed (Fig. 2a, lane 6), indicating that patients with Chagas disease are able to make antibodies against rTcTIM. This observation was further explored in a Western blot using individual sera from four patients with Chagas disease. Fig. 2b shows that all four individual sera recognize the 27 kDa band, albeit with different intensities (lanes 2–5). Normal human serum does not recognize rTcTIM, as shown in lane 6. The recognition of rTcTIM by sera of patients with Chagas disease provides evidence that this protein is immunogenic.
Electrophoretic analysis and Western blot analysis of lysates of T. cruzi epimastigotes, rTcTIM and human recombinant TIM using the mAb (6-11G) or sera from patients with Chagas disease. a Lanes 1–3 are proteins stained with Coomassie blue, whereas lanes 4–6 are Western blots. Lane 1 Low-range MW standards Biorad; lane 2 T. cruzi lysate proteins; lane 3 rTcTIM protein; lane 4 Western blot of whole parasite lysate with mAb 6-11G; lane 5 Western blot of rTcTIM with mAb 6-11G; lane 6 Western blot of rTcTIM with a pool of sera from patients with Chagas disease. b Western blot analysis of rTcTIM using four individual sera from patients with Chagas disease (lanes 2–5) and normal human control serum (lane 6). c Western blot analysis of human recombinant TIM using sera from patients with Chagas disease (lanes 2–4), normal human serum (lanes 5 and 6), mAb 6-11G (lane 7) and normal mouse serum (lane 8). a Even though the parasite lysate presents a large amount of protein bands (lane 2), the mAb 6-11G is very specific since it only recognizes a protein of 27 kDa in the Western blot (lane 4). This is confirmed when analyzing the rTcTIM, which has a weight of 27 kDa (lane 3). The Western blot of rTcTIM shows that the mAb recognizes this 27 kDa protein (lane 5). Sera (pool) from patients with Chagas disease also recognize rTcTIM (lane 6). b Individual sera from four patients with Chagas disease recognize rTcTIM (lanes 2–4), whereas normal human serum does not (lane 6). c Sera from patients with Chagas disease (lanes 2–4) and mAb 6-11G also recognize human recombinant TIM (lane 7), whereas normal human serum (lanes 5 and 6) and normal mouse serum (lane 8) show no recognition
Additionally, to ascertain if there is cross-reactivity between rTcTIM and human recombinant TIM, we analyzed if sera from patients with Chagas disease and the mAb 6-11G also recognizes human recombinant TIM (Fig. 2c). A Western blot using recombinant human TIM showed that it was recognized by sera from patients with Chagas disease (lanes 2–4) as well as by the mAb 6-11G (lane 7). Normal human sera showed no recognition (lanes 5 and 6), nor did nonrelated mAbs (lane 8). Thus, this finding indicates that anti-T. cruzi TIM antibodies of patients with Chagas disease cross-react with human TIM.
Distribution of TIM in epimastigotes of T. cruzi
To probe into the mechanism through which mAb inhibits the growth of T. cruzi epimastigotes, we examined the distribution of TcTIM in their cellular space. To this end, TcTIM was immunolocalized in epimastigotes that had been fixed in cold acetone, for immunofluorescent staining or in glutaraldehyde for electron microscopic studies.
Immunofluorescent staining of TIM in T. cruzi epimastigotes
mAb (6-11G) and a secondary anti-mouse IgG antibody coupled to fluorescein isothiocyanate were used for the identification of TIM in T. cruzi epimastigotes. Parasites showed intense immunofluorescent staining in cytoplasmic dots, nuclei, kinetoplasts and faintly in the cytosol (Fig. 3a). Occasionally, flagella were also stained.
Immunolocalization of TIM in T. cruzi epimastigotes using the mAb 6-11G for immunofluorescence and for immunogold with electron microscopy. Immunofluorescence shows staining in cytoplasmic dots, nuclei, kinetoplasts, and faintly in the cytosol (a). Immunogold staining shows that the label is seen in the nucleus (N), the kinetoplast (K), in clear vesicles and throughout the cytosol (b). No specific label is observed with the nonrelated monoclonal antibody (c). Gold particles were also observed inside organelles which exhibited a dense core, frequently displaying a multillamelar appearance (d, arrows). (a bar 5 μm; b: bar 500 nm; c bar 500 nm; d bar 250 nm)
The ultrastructural localization of TIM in T. cruzi epimastigotes was further explored with an immunogold postembedding protocol with the same primary antibody and a secondary antibody coupled to colloidal gold. As shown in Fig. 3b, the label is seen in the nucleus, the kinetoplast, in clear vesicles, and throughout cytosol. Gold particles were also observed inside of single thick structures of spherical shape bounded by membranes with diameters ranging approximately from 200 to 500 nm. Many of these organelles exhibited a dense core, frequently displaying a multillamelar appearance (Fig. 3d, arrows). Negative controls using a nonrelated monoclonal antibody showed no specific label (Fig. 3c).
To establish whether the immunogold staining of these multilamellar or dense-cored vesicles in T. cruzi epimastigotes corresponded to glycosomes, we purified T. brucei glycosomes. Since mAb 6-11G recognizes both rTcTIM and rTbTIM, we stained the purified glycosomes with this antibody. As can be seen in Fig. 4, numerous purified glycosomes of T. brucei contain one or more gold particles over the dense or crystalloid core displaying a lamellar structure. These fine structural features, in addition to the ultrastructural immunolocalization of TIM, confirmed that purified microbodies from T. brucei are glycosomes and that they are homologous to the labeled dense-cored organelles observed in T. cruzi epimastigotes (see Fig. 3d). Negative controls using a nonrelated monoclonal antibody showed no staining (data not shown).
Discussion
We produced a monoclonal antibody against TcTIM that inhibited growth and survival of T. cruzi epimastigostes after 24 h of culture. This finding is in accordance with data obtained with other pathogenic parasites, where the production of antibodies against TIM has proven beneficial to the host. For example, in Schistosoma mansoni infections, a mAb against TIM induced partial inhibition of the enzyme and passive resistance in mice that received the mAb prior to the infection with S. mansoni (Harn et al. 1991). Likewise, it has been shown that immunization against Taenia solium with TIM protects mice against infection with Taenia crassiceps (Jiménez et al. 2003). Our data show that T. cruzi TIM is also an immunogenic molecule, since it was recognized by sera from patients with Chagas disease (Fig. 2a, lane 6; Fig. 2b, lanes 2–4). However, the antibodies against TcTIM detected in patients with Chagas disease also recognized human TIM (Fig. 2c, lanes 2–4). This was an unexpected finding and questions the applicability of T. cruzi TIM for immunization and protection against Chagas disease, although it has been used successfully in other parasitic diseases. In T. cruzi infections, the formation of antibodies against TcTIM seems to be a double-edged sword, since on one side, it could limit parasite growth and survival, but on the other hand, the antibodies could cross-react with host TIM, contributing to the autoimmune disease mechanisms that are considered a hallmark in Chagasic pathology (Kierszenbaum 2005).
The finding that the mAb against T. cruzi TIM induced parasite death led us to analyze the location of TIM in T. cruzi epimastigotes to explore how the mAb produces such effect. Our ultrastructural studies revealed that there was no apparent binding of mAb to the cell surface; instead, they showed that TcTIM is present in the glycosomes, which is in line with several reports that indicate that glycosomes have substantial amounts of TIM; in fact, in T. brucei, the available evidence indicates that TbTIM exists exclusively in the glycosomes (Opperdoes and Borst 1977).
It is remarked, however, that our ultrastructural studies also showed that the enzyme exists in the nucleus, the cytosol, and the kinetoplast. In this context, it is relevant that Bourgignon et al. (1998), using a polyclonal rabbit against TcTIM, observed that TcTIM was in the cytosol; albeit their electron microscope techniques did not allow them to ascertain if the enzyme localized exclusively in the glycosomes. Nonetheless, and along this line, it is relevant that Taylor and Gutteridge (1987) studied the subcellular location of glycolytic enzymes in T. cruzi; they found by isopycnic centrifugation that TcTIM was indeed in the glycosomes; however, they also found that a significant portion of the enzyme was in the lighter fractions, including the soluble fraction. Thus, the biochemical data of Taylor and Gutteridge (1987) and our ultrastructural studies on the location of TcTIM are in full agreement and support the notion that TcTIM has multiple locations in T. cruzi epimastigotes.
Taylor and Gutteridge (1987) also reported that the first six enzymes of the glycolytic pathway, including TcTIM, are present in the glycosomal fraction of T. cruzi epimastigotes. Thus, it is very likely that in T. cruzi, and similarly to T. brucei, this portion of glycolysis pathway is carried out in the glycosomes. Thus, the question that arises from the data of Taylor and Gutteridge (1987) and from our own data concerns the function of TcTIM that is localized in the nonglycosomal fraction. This question acquires significant relevance particularly because mAb is deleterious to T. cruzi epimastigotes.
Since in lysates of epimastigotes, mAb recognized only the protein band that corresponds to TcTIM, it may be safely assumed that mAb is highly specific. Thus, in principle, the detrimental effect of mAb could be due to its interaction with glycosomal TcTIM, with the enzymes that lay outside of the glycosome, or with enzymes that exists in both fractions. At the moment, this question cannot be answered. However, if the detrimental effect of mAb is due to its interaction with nonglycosomal TcTIM, it would appear that the function of nonglycosomal TcTIM is central in the life of the parasite.
In conclusion, the present findings shed new light on the vital importance of this enzyme for T. cruzi survival and opens new perspectives for parasite-drug development. Additionally, we report a possible novel molecule that contributes to the autoimmune Chagasic pathology.
References
Aguirre-García MM, Cerbon J, Talamás-Rohana P (2000) Purification and properties of an acid phosphatase from Entamoeba histolytica HM-1 IMSS. Int J Parasitol 30:585–591
Aman RA, Wang CC (1986) An improved purification of glycosomes from the procyclic trypomastigotes of Trypanosoma brucei. Mol Biochem Parasitol 21:211–220
Berriman M, Ghedin E, Fowler CH, Blandin G, Renauld H, Bartholomeu DC, Lennard NJ, Caler E, Hamlin NE, Haas B, Böhme U, Hannick L, Aslett MA, Shallom J, Marcello L, Hou L, Wickstead B, Alsmark UCM, Arrowsmith C, Atkin RJ, Barron AJ, Bringaud F, Brooks K, Carrington M, Cherevach I, Chillingworth TJ, Churcher C, Clark LN, Corton CH, Cronin A, Davies RM, Doggett J, Djikeng A, Feldblyum T, Field MC, Fraser A, Goodhead I, Hance Z, Harper D, Harris BR, Hauser H, Hostetler J, Ivens AL, Jagels K, Johnson D, Johnson J, Jones K, Kerhornou AX, Koo H, Larke N, Landfear S, Larkin C, Leech V, Line A, Lord A, Macleod A, Mooney PJ, Moule S, Martin DMA, Morgan GW, Mungal LK, Norbertczak H, Ormond D, Pai G, Peacock CS, Peterson J, Quail MA, Rabbinowitsch E, Rajandream MA, Reiter C, Salzberg SL, Sanders M, Schobel S, Sharp S, Simmonds M, Simpson AJ, Tallon L, Turner CMR, Tait A, Tivey AR, Aken SV, Walker D, Wanless D, Wang S, White B, White O, Whitehead S, Woodward J, Wortman J, Adams MD, Embley TM, Gull K, Ullu E, Barry JD, Fairlamb AH, Opperdoes F, Barrell BG, Donelson JE, Hall N, Fraser CM, Melville SE, El-sayed NM (2005) The genome of the African trypanosome Trypanosoma brucei. Science 309:416–422
Bourgignon SC, Meirelles MN, Pacheco RS, Giovanni DS (1998) Purification and partial characterization of Trypanosoma cruzi triosephosphate isomerase. Mem Inst Oswaldo Cruz 93:219–224
Bozzola JJ, Russell LD (1998) Electron microscopy, 2nd edn. Jones and Bartlett Publishers, Sudbury, MS
El-Sayed NM, Myler PJ, Bartholomeu DC, Nilsson D, Aggarwal G, Tran AN, Ghedin E, Worthey EA, Delcher AL, Blandin G, Westenberger SJ, Caler E, Cerqueira GC, Branche C, Haas B, Anupama A, Arner E, Aslund L, Attipoe P, Bontempi E, Bringaud F, Burton P, Cadag E, Campbell DA, Carrington M, Crabtree J, Darban H, Da silveira JF, De jong P, Edwards K, Englund PT, Fazelina G, Feldblyum T, Ferella M, Frasch AC, Gull K, Horn D, Hou L, Huang Y, Kindlund E, Klingbeil M, Kluge S, Koo H, Lacerda D, Levin MJ, Lorenzi H, Louie T, Machado CR, Mc Culloch R, McKenna A, Mizuno Y, Mottram JC, Nelson S, Ochaya S, Osoegawa K, Pai G, Parsons M, Pentony M, Pettersson U, Pop M, Ramirez JL, Rinta J, Robertson L, Salzberg SL, Sanchez DO, Seyler A, Sharma R, Shetty J, Simpson AJ, Sisk E, Tammi MT, Tarleton R, Teixeira S, Aken AV, Vogt C, Ward PN, Wickstead B, Wortman J, White O, Fraser CM, Stuart KD, Andersson B (2005) The genome sequence of Trypanosoma cruzi, etiological agent of Chagas disease. Science 309:409–415
Hannaert V, Michels PA (1994) Structure, function and biogenesis of glycosomes in kinetoplastida. J Bioenerg Biomembr 26:205–212
Harn DA, Gu W, Oligino LD, Mitsuyama M, Gebremichael A, Richter D (1991) Protective monoclonal antibody specifically recognizes and alters the catalytic activity of Schistosome triose-phosphate isomerase. J Immunol 148:562–567
Hart DT, Opperdoes FR (1984) The occurrence of glycosomes (microbodies) in the promastigote stage of four major Leishmania species. Mol Biochem Parasitol 13:159–172
Ivens AC, Peacock CS, Worthey EA, Murphy L, Aggarwal G, Berriman M, Sisk E, Rajandream MA, Adlem E, Aert R, Anupama A, Apostolou Z, Attipoe P, Bason N, Bauser C, Beck A, Beverley SM, Bianchettin G, Borzym K, Bothe G, Bruschi CV, Collins M, Cadag E, Ciarloni L, Clayton C, Coulson RMR, Cronin A, Cruz AK, Davies RM, De Gaudenzi J, Dobson DE, Duesterhoeft A, Fazelina G, Fosker N, Frasch CA, Fraser A, Fuchs M, Gabel C, Goble A, Goffeau A, Harris D, Hertz-Fowler C, Hilbert H, Horn D, Huang Y, Klages S, Knights A, Kube M, Larke N, Litvin L, Lord A, Louie T, Marra M, Masuy D, Matthews K, Michaeli S, Mottram JC, Müller-Auer S, Munden H, Nelson S, Norbertczak H, Oliver K, O’Neil S, Pentony M, Pohl TM, Price C, Purnelle B, Quail MA, Rabbinowitsch E, Reinhardt R, Rieger M, Rinta J, Robben J, Robertson L, Ruiz JC, Rutter S, Saunders D, Schäfer M, Schein J, Schwartz DC, Seeger K, Seyler A, Sharp S, Shin H, Sivam D, Squares R, Squares S, Tosato V, Vogt C, Volckaert G, Wambutt R, Warren T, Wedler H, Woodward J, Zhou S, Zimmermann W, Smith DF, Blackwell JM, Stuart KD, Barrell B, Myler PJ (2005) The genome of the kinetoplastid parasite Leishmania major. Science 309:436–442
Jiménez L, Fernández-Velasco DA, Willms K, Landa A (2003) A comparative study of biochemical and immunological properties of triosephosphate isomerase from Taenia solium and Sus scrofa. J Parasitol 89:209–214
Kierszenbaum F (2005) Where do we stand on the autoimmune hypothesis of Chagas disease? Trends Parasitol 21:513–516
Lanham SM (1968) Separation of trypanosomes from blood of infected rats and mice by anion-exchangers. Nature 218:1273–1274
Nozaki T, Engel JC, Dvorak JA (1996) Cellular and molecular biological analysis of nifurtimox resistance in Trypanosoma cruzi. Am J Trop Med. Hyg 55:111–117
Opperdoes FR (1987) Compartmentation of carbohydrate metabolism in trypanosomes. Ann Rev Microbiol 41:127–151
Opperdoes FR, Borst P (1977) Localization of nine glycolytic enzymes in a microbody-like organelle in Trypanosoma brucei: The glycosome. FEBS Lett 80:360–364
Opperdoes FR, Michels PA (1993) The glycosomes of the Kinetoplastida. Biochemie 75:231–234
Opperdoes FR, Baudhuin P, Coppens I, De Roe C, Edwards SW, Wiejers PJ, Misset O (1984) Purification, morphometric analysis, and characterization of the glycosomes (microbodies) of the protozoan hemoflagellate Trypanosoma brucei. J Cell Biol 98:1178–1184
Ostoa-Saloma P, Garza-Ramos G, Ramírez J, Becker I, Berzunza M, Landa A, Gómez- Puyou A, Pérez-Montfort R (1997) Cloning, expression, purification and characterization of triosephosphate isomerase from Trypanosoma cruzi. Eur J Biochem 244:700–705
Sambrook J, Fritsch E, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Taylor MB, Gutteridge WE (1987) Trypanosoma cruzi: subcellular distribution of glycolytic and some related enzymes of epimastigotes. Exp Parasitol 63:84–97
Taylor MB, Berghausen H, Heyworth P, Messenger N, Rees LJ, Gutteridge WE (1980) Subcellular localization of some glycolytic enzymes in parasitic flagellated protozoa. Int J Biochem 11:117–120
Urbina JA, Payares G, Molina J, Sanoja C, Liendo A, Lazardi K, Piras MM, Piras R, Perez N, Wincker P (1996) Cure of short- and long-term experimental Chagas disease using D0870. Science 273:969–971
Visser N, Opperdoes FR (1980) Glycolysis in Trypanosoma brucei. Eur J Biochem 103:623–632
WHO (1991) Expert Committee on Control of Chagas Disease. WHO Techn. Rep. Ser. No. 811. Geneva
Zimmermann S, Becker-Pérez I, Beuscher HU, Kroczek RA, Röllinghoff M, Solbach W (1994) Leishmania major parasites share an epitope with the murine CD3-T complex. Eur J Immunol 24:503–507
Acknowledgments
We thank Rocely Cervantes Sarabia, Miriam Berzunza Cruz, Adriana Ruiz Remigio, and Marco Gudiño Zayas for technical assistance and Lucía Alvarez Trejo for excellent secretarial support. The experiments comply with the current laws of Mexico, and all animal studies were carried out following the guidelines of ethical treatment for animal care established in the country. This work was funded by grants CONACyT: 47256-M and DGAPA: IN 221806. AA Cortés-Figueroa is recipient of a CONACyT fellowship for the Posgrado en Ciencias Biomédicas, Universidad Nacional Autónoma de México.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cortés-Figueroa, A., Pérez-Torres, A., Salaiza, N. et al. A monoclonal antibody that inhibits Trypanosoma cruzi growth in vitro and its reaction with intracellular triosephosphate isomerase. Parasitol Res 102, 635–643 (2008). https://doi.org/10.1007/s00436-007-0803-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-007-0803-5