Abstract
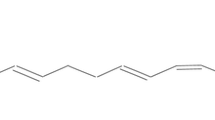
Glutathione S-transferase activity has been shown to be associated with the microsomal fraction of Taenia solium. Electron microscopy and subcellular enzyme markers indicate the purity of the microsomal fraction that contains the glutathione S-transferase activity. T. solium microsomes were solubilized under conditions used to solubilize integral microsomal proteins. This procedure proved necessary to obtain enzymatic activity. To characterize this parasite enzyme activity, several substrates and inhibitors were used. The optimum activity for microsomal glutathione S-transferase was found to be pH 6.6, with a specific enzyme activity of 0.9, 0.1, 0.067, 0.03, and 0.05 μmol min−1 mg−1 with the substrates 1-chloro-2,4-dinitrobenzene (CDNB), 1,2-dichloro-4-nitrobenzene, 4-hydroxynonenal, 2,4-hexadienal, and trans-2-nonenal, respectively. No activity of glutathione peroxidase was observed. T. solium microsomes had an app K m (GSH) = 0.161 μM, app K m (CDNB) = 14.5 μM, and app V max of 0.15 and 27.9 μmol min−1 mg−1 for GSH and CDNB, respectively. T. solium microsomes were inhibited by several glutathione S-transferase enzyme inhibitors, and it was possible to establish a simple inhibition system as well as corresponding K i ’s for each inhibitor. These results indicate that the T. solium microsomal glutathione S-transferase is different from the parasite cytoplasmic enzymes that catalyze similar reactions.




Similar content being viewed by others
References
Anderson C, Soderstrom M, Mannervick B (1988) Activation and inhibition of microsomal glutathione S-transferase from mouse liver. Biochem J 249:819–823
Angeli V, Faveeuw Ch, Roye O, Fontaine J, Teissier E, Capron A, Wolowczuk I, Capron M, Trottein F (2001) Role of the parasite-derived prostaglandin D2 in the inhibition of epidermal Langerhans cell migration during Schistosomiasis infection. J Exp Med 193:1135–1147
Arrigoni O, Singer TP (1962) Limitations of the phenazine methosulfate assay for succinic and related dehydrogenase. Nature (London) 193:1256–1258
Baars AJ, Jansen M, Breimer D (1979) Xenobiotic metabolizing enzymes in Drosophila melanogaster. Activities of epoxide hydratasa and glutathione S-transferase compared with similar activities in rat liver. Mutation Res 62:279–291
Baset HA, O’Neill GP, Ford-Hutchinson AW (1995) Characterization of arachidonic-acid-metabolizing enzymes in adult Schistosoma mansoni. Mol Biochem Parasitol 73:31–41
Belley A, Chadee K (1995) Eicosanoid production by parasites: from pathogenesis to immunomodulation? Parasitol Today 11:327–334
Bresell A, Weinander R, Lundqvist G, Raza H, Shimoji M, Sun T, Balk L, Wiklund R, Ericsson J, Jansson Ch, Peterson B, Jakobsson P (2005) Bioinformatic and enzymatic characterization of the MAPEG superfamily. Fed Eur Biochem Soc J 272:1688–1703
Brophy PM, Southan C, Barret J (1989) Glutathione transferases in the tapeworm Moniezia expansa. Biochem J 262:939–946
Brophy PM, Barret J (1990) Glutathione transferase in helminthes. Parasitology 100:345–349
Clemens JA, Ho PP, Panetta JA (1991) LY178002 reduces rat brain damage after transient global forebrain ischemia. Stroke 22:1048–1052
Cochrane BJ, Morrissey JJ, LeBlanc GA (1987) The genetics of xenobiotic metabolism in Drosophila. IV. Purification and characterization of the major glutathione S-transferase. Insect Biochem 17:731–738
Danielson PUH, Mannervik B (1985) 4-hydroxyalk-2-enals are substrates for transferase. FEBS 179:267–270
deDuve C, Pressman BC, Gianetto R, Wattiaux R, Appelmans F (1955) Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J 60:604–617
Dierickx PJ (1987) Soluble glutathione S-transferase isoenzymes in Daphnia magna strains and their interactions with 2,4-dichlorophenoxy acetic acid and 1,4-benzoquinone. Insect Biochem 17:1–6
Dixon HBF, Lipscomb FM (1961) Cysticercosis: an analysis and follow up of 450 cases. Privy Council Med Res Spec Rep Ser Lond 229:1–58
Emerit I (1987) Clastogenic factors, a link between chronic inflammation and carcinogenesis. In: Cerutti PA, Nygaard OF, Simic MG (eds) Anticarcinogenesis and radiation protection. Plenum, New York, pp 59–62
Ernster L, Siekevitz P, Palade G (1962) Enzyme–structure relationships in the endoplasmic reticulum of rat liver. A morphological and biochemical study. J Cell Biol 15:541–562
Esterbauer H, Benedetti S, Lang J, Fulceri R, Fauler G, Comporti M (1986) Studies on the mechanism of formation of 4-hydroxynonenal during microsomal lipid peroxidfation. Biochem Biophys Acta 876:154–166
Fiske CH, SubbaRow Y (1925) The colorimetric determination of phosphorus. J Biol Chem 66:375–400
Fleury A, Gomez T, Alvarez I, Meza D, Huerta M, Chavarria A, Carrillo Mezo RA, Lloyd C, Dessein A, Preux PM, Dumas M, Larralde C, Sciutto E, Fragoso G (2003) High prevalence of calcified silent neurocysticercosis in a rural village of Mexico. Neuroepidemiology 22:130–145
Friedberg T, Bentley Ph, Stasiecki P, Glatt HR, Raphael D, Oesch F (1979) The identification, solubilization, and characterization of microsome-associated glutathione S-transferases. J Biol Chem 254:12028–12033
Fusco AC, Salafsky B, Delbrook K (1986) Schistosoma mansoni. : production of cercarial eicosanoids as correlates of penetration and transformation. J Parasitol 72:397–404
Gonzalez R, Mendoza-Hernandez G, Plancarte A (2002) Purification of Taenia solium cysticerci superoxide dismutase and myoglobin copurification. Parasitol Res 88:881–887
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferase. The first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Jakobsson P, Morgenstern R, Mancini J, Ford-Hutchinson A, Persson B (2000) Membrane-associated proteins in eicosanoid and glutathione metabolism (MAPEG). A widespread protein superfamily. Am J Resp Crit Care Med 161:S20–S24
Jakoby WB (1978) The glutathione S-transferases: a group of multifunctional detoxification proteins. Adv Enzymol 46:383–414
Josephy PD, Mannervik B, Ortiz de Montellano P (1997) Carcinogenic: the genetic target. In: Josephy PD, Mannervik B, Ortiz de Montellano P (eds) Molecular toxicology. University Press, Oxford, UK, pp 309–315
Kozumbo W, Muehiematter D, Ochi T, Cerutti P (1987) The role of active oxygen and the metabolism of arachidonic acid in the formation of clastogenic factor by human monocytes. In: Cerutti PA, Nygaard OF, Simic MG (eds) Anticarcinogenesis and radiation protection. Plenum, New York, pp 51–57
Leid RW, McConnell LA (1983) PGE2 generation and release by the larvae stage of the cestode, Taenia taeniaeformis. Prostaglandins Leukot Med 11:317–323
Leid RW, Suquet CM, Taniagoshi L (1987) Parasite defense mechanism for evasion of host attack: a review. Vet Parasitol 25:147–162
Morgenstern R, DePierre W (1983) Purification in unactivated form and further characterization of the activation process, substrate specificity and amino acid composition. Eur J Biochem 134:591–597
Morgenstern R, DePierre JW, Ernster L (1979) Activation of microsomal glutathione S-transfrease activity by sulfhydryl reagents. Biochem Biophys Res Commun 87:657–663
Morgenstern R, Meijer J, DePierre JW, Ernster L (1980) Characterization of rat-liver microsomal glutathione S-transferase activity. Eur J Biochem 104:167–174
Morgenstern R, Lundqvist G, Hancock V, DePierre W (1988) Studies on the activity and activation of rat liver microsomal glutathione S-transferase, in particular with a substrate analogue series. J Biol Chem 263:6671–6675
Mosialou E, Morgenstern R (1989) Activity of rat liver microsomal glutathione transferase toward products of lipid peroxidation and studies of the effect of inhibitors on glutathione-dependent protection against lipid peroxidation. Arch Biochem Biophys 275:289–294
Mosialou E, Morgenstern R (1990) Inhibition studies on rat liver microsomal glutathione transferase. Chem Biol Interact 74:275–280
Mosialou E, Piemonte F, Andersson C, Vos REM, van Bladeren P, Morgenstern R (1995) Microsomal glutathione transferase: lipid-derived substrates and lipid dependence. Arch Biochem Biophys 320:210–216
O’Leary KA, Hathaway KM, Tracy JW (1992) Schistosoma mansoni. : single step purification and characterization of glutathione S-transferase isoenzyme 4. Exp Parasitol 75:47–55
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–169
Peterson GL (1977) A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem 83:346–356
Plancarte A, Rendón JL, Landa A (2004) Purification, characterization and kinetic properties of the Taenia solium glutathione S-transferase isoform 26.5 kDa. Parasitol Res 93:137–144
Prabhakar S, Singh G (2002) Neurocysticercosis: an overview of clinical presentation. In: Singh G, Prabhakar S (eds) Taenia solium cysticercosis, from basic to clinical science. CABI, Oxford, UK, pp 169–176
Schantz PM (2002) Taenia solium cysticercosis: an overview of global distribution and transmission. In: Singh G, Prabhakar S (eds) Taenia solium cysticercosis, from basic to clinical science. CABI, Oxford, UK, pp 63–73
Segel I (1975) Simple inhibition systems. In: Segel I (ed) Enzyme kinetics. Behavior and analysis of rapid equilibrium and steady-state enzyme systems. Wiley, New York, p 957
Shandera WX, White AC, Chen J, Diaz P, Armstrong R (1994) Cysticercosis in Houston, Texas: a report of 112 cases. Medecine 73:37–52
Spurr AR (1969) A low viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res 26:31
Swanson MA (1955) Glucose-6-phosphatase from liver. Methods Enzymol 2:541–543
Tahir MK, Mannervik B (1985) Inhibitors for distinction of three types of human glutathione transferase. FEBS Lett 181:249–252
Tahir MK, Mannervik B (1986) Simple inhibition studies for distinction between homodimeric and heterodimeric isoenzymes of glutathione transferase. J Biol Chem 261:1048–1051
Vassault A (1983) UV-method with pyruvate and NADH. In: Bergmeyer HUB, Bergmeyer J, Grabe M (eds) Methods in enzymatic analysis, vol. III. Academic, New York, pp 118–126
Vibanco-Perez N, Jiménez L, Mendoza-Hernandez G, Landa A (2002) Characterization of a recombinant mu-class glutathione S-transferase from Taenia solium. Parasitol Res 88:398–404
Weitberg AB, Weitzman SA, Destremps M, Latt SA, Stossel TP (1983) Stimulated human phagocytes produce of cytogenic changes in cultured mammalian cells. New Eng J Med 308:26–30
Acknowledgments
The authors wish to thank Dr. Gerardo Medina for valuable English corrections. We thank Dra. Irene P. del Arenal, who kindly assisted us during the succinate dehydrogenase measurement. The present study was funded by grant from Universidad Nacional Autónoma de México, DGAPA (PAPIIT-IN201906-3). All the experiments complied with the current laws of the Universidad Nacional Autónoma de México.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nava, G., Robert, L. & Plancarte, A. Characterization of Taenia solium cysticerci microsomal glutathione S-transferase activity. Parasitol Res 101, 1373–1381 (2007). https://doi.org/10.1007/s00436-007-0655-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-007-0655-z