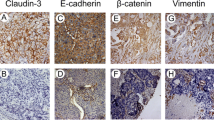
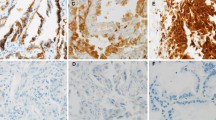
Abstract
Background
Pulmonary invasive mucinous adenocarcinoma (IMA) is a unique type of lung adenocarcinoma with a high recurrence rate and limited treatment strategies. The tight-junction-associated protein claudin18.2 is a new therapeutic target for several solid tumors. This study aimed to detect the expression of claudin18.2 in IMA and its clinicopathological association with the disease.
Methods
The expression of claudin18.2 was immunohistochemically evaluated in an IMA cohort of 84 patients, who underwent partial pneumonectomy between January 2017 and December 2021. Positive staining for claudin18.2 was defined as ≥ 10% of tumor cells showing ≥ 1 + membrane staining or any ≥ 2 + membrane staining.
Results
Claudin18.2 was detected in 76.2% (64/84) of IMA patients, significantly higher than that in non-mucinous adenocarcinoma (NMA). 46.4% (39/84) of the IMA patients met the enrollment criteria of the clinical trials of monoclonal antibodies (≥ 75% of tumor cells demonstrating ≥ 2 + staining intensity). Positive staining for claudin18.2 was significantly associated with smaller tumor size (p = 0.010), less pleural invasion (p = 0.019), and earlier pN stage (p < 0.001). Expression of claudin18.2 was not related to prognosis in multivariate analysis.
Conclusions
To summarize, in this study we found that claudin18.2 was remarkably highly expressed in IMA and the overexpression was associated with low invasive capacity. Thus, this protein appears to be a promising therapeutic target and deserves further investigation in IMA patients.



Similar content being viewed by others
Data availability
The data analyzed during the current study are available from the corresponding author on reasonable request.
References
Arpa G, Fassan M, Guerini C, Quaquarini E, Grillo F, Angerilli V, Guzzardo V, Lonardi S, Bergamo F, Lenti MV, Pedrazzoli P, Paulli M, Di Sabatino A, Vanoli A (2022) Claudin-18 expression in small bowel adenocarcinoma: a clinico-pathologic study. Virchows Arch 481(6):853–863
Baek JH, Park DJ, Kim GY, Cheon J, Kang BW, Cha HJ, Kim JG (2019) Clinical Implications of Claudin18.2 Expression in Patients With Gastric Cancer. Anticancer Res 39(12):6973–6979
Cao W, Xing H, Li Y, Tian W, Song Y, Jiang Z, Yu J (2022) Claudin18.2 is a novel molecular biomarker for tumor-targeted immunotherapy. Biomark Res 10(1):38
Duruisseaux M, Antoine M, Rabbe N, Rodenas A, Mc Leer-Florin A, Lacave R, Poulot V, Duchene B, Van Seuningen I, Cadranel J, Wislez M (2017) Lepidic predominant adenocarcinoma and invasive mucinous adenocarcinoma of the lung exhibit specific mucin expression in relation with oncogenic drivers. Lung Cancer 109:92–100
Guo M, Tomoshige K, Meister M, Muley T, Fukazawa T, Tsuchiya T, Karns R, Warth A, Fink-Baldauf IM, Nagayasu T, Naomoto Y, Xu Y, Mall MA, Maeda Y (2017) Gene signature driving invasive mucinous adenocarcinoma of the lung. EMBO Mol Med 9(4):462–481
Iwaya M, Hayashi H, Nakajima T, Matsuda K, Kinugawa Y, Tobe Y, Tateishi Y, Iwaya Y, Uehara T, Ota H (2021) Colitis-associated colorectal adenocarcinomas frequently express claudin 18 isoform 2: implications for claudin 18.2 monoclonal antibody therapy. Histopathology 79(2):227–237
Kominsky SL (2006) Claudins: emerging targets for cancer therapy. Expert Rev Mol Med 8(18):1–11
Liu J, Yang H, Yin D, Jia Y, Li S, Liu Y (2022) Expression and prognostic analysis of CLDN18 and Claudin18.2 in lung adenocarcinoma. Pathol Res Pract 238:154068
Lordick F, Al-Batran SE, Ganguli A, Morlock R, Sahin U, Tureci O (2021) Patient-reported outcomes from the phase II FAST trial of zolbetuximab plus EOX compared to EOX alone as first-line treatment of patients with metastatic CLDN18.2+ gastroesophageal adenocarcinoma. Gastric Cancer 24(3):721–730
Luo J, Chimge NO, Zhou B, Flodby P, Castaldi A, Firth AL, Liu Y, Wang H, Yang C, Marconett CN, Crandall ED, Offringa IA, Frenkel B, Borok Z (2018) CLDN18.1 attenuates malignancy and related signaling pathways of lung adenocarcinoma in vivo and in vitro. Int J Cancer 143(12):3169–3180
Matsuda M, Sentani K, Noguchi T, Hinoi T, Okajima M, Matsusaki K, Sakamoto N, Anami K, Naito Y, Oue N, Yasui W (2010) Immunohistochemical analysis of colorectal cancer with gastric phenotype: claudin-18 is associated with poor prognosis. Pathol Int 60(10):673–680
Micke P, Mattsson JSM, Edlund K, Lohr M, Jirström K, Berglund A, Botling J, Rahnenfuehrer J, Marincevic M, Pontén F, Ekman S, Hengstler J, Wöll S, Sahin U, Türeci Ö (2014) Aberrantly activated claudin 6 and 18.2 as potential therapy targets in non-small-cell lung cancer. Int J Cancer 135(9):2206–2214
Sahin U, Koslowski M, Dhaene K, Usener D, Brandenburg G, Seitz G, Huber C, Türeci Ö (2008) Claudin-18 splice variant 2 is a pan-cancer target suitable for therapeutic antibody development. Clin Cancer Res 14(23):7624–7634
Sahin U, Türeci Ö, Manikhas G, Lordick F, Rusyn A, Vynnychenko I, Dudov A, Bazin I, Bondarenko I, Melichar B, Dhaene K, Wiechen K, Huber C, Maurus D, Arozullah A, Park JW, Schuler M, Al-Batran S-E (2021) FAST: a randomised phase II study of zolbetuximab (IMAB362) plus EOX versus EOX alone for first-line treatment of advanced CLDN18.2-positive gastric and gastro-oesophageal adenocarcinoma. Ann Oncol 32(5):609–619
Shimobaba S, Taga S, Akizuki R, Hichino A, Endo S, Matsunaga T, Watanabe R, Yamaguchi M, Yamazaki Y, Sugatani J, Ikari A (2016) Claudin-18 inhibits cell proliferation and motility mediated by inhibition of phosphorylation of PDK1 and Akt in human lung adenocarcinoma A549 cells. Biochim Biophys Acta 1863(6 Pt A):1170–1178
Türeci Ö, Koslowski M, Helftenbein G, Castle J, Rohde C, Dhaene K, Seitz G, Sahina U (2011) Claudin-18 gene structure, regulation, and expression is evolutionary conserved in mammals. Gene 481(2):83–92
Wang X, Zhang C-S, Dong X-Y, Hu Y, Duan B-J, Bai J, Wu Y-Y, Fan L, Liao X-H, Kang Y, Zhang P, Li M-Y, Xu J, Mao Z-J, Liu H-T, Zhang X-L, Tian L-F, Li E-X (2022) Claudin 18.2 is a potential therapeutic target for zolbetuximab in pancreatic ductal adenocarcinoma. World J Gastrointest Oncol 14(7):1252–1264
Xu B, Liu F, Liu Q, Shi T, Wang Z, Wu N, Xu X, Li L, Fan X, Yu L, Liu B, Wei J (2020) Highly expressed Claudin18.2 as a potential therapeutic target in advanced gastric signet-ring cell carcinoma (SRCC). J Gastrointest Oncol 11(6):1431–1439
Yoshizawa A, Motoi N, Riely GJ, Sima CS, Gerald WL, Kris MG, Park BJ, Rusch VW, Travis WD (2011) Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol 24(5):653–664
Funding
This study is supported by the National High Level Hospital Clinical Research Funding 2023-PUMCH-F-004 (D.G.), 2022-PUMCH-B-062 (J.S.), 2022-PUMCH-D-002 (D.G) and CAMS Innovation Fund for Medical Sciences (CIFMS) 2021-I2M-1-053 (D.G. and Z.-Y.L.).
Author information
Authors and Affiliations
Contributions
YMW, ZWZ, and YKG gathered the cohort, interpreted the immunohistochemical staining, and analyzed the clinical and pathology data. YMW visualized the data and wrote the original draft. ZWZ and YKG edited the manuscript. ZXZ, AQW, KZ, and MZ collected materials, prepared the sections, and performed immunohistochemical staining. SMZ participated in the pre-study of tissue microarrays. JS and DG participated in the study design and supervision. ZYL provided resources and reviewed the study. All authors discussed the results and contributed to the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests with respect to the research.
Ethics approval
This study was approved by the Institutional Review Board of Peking Union Medical College Hospital (protocol number: JS-3553) and all the research was performed in accordance with relevant guidelines.
Informed consent
Informed consent was obtained for all patients enrolled in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Y., Gao, Y., Zhang, Z. et al. Claudin18.2 expression in pulmonary mucinous adenocarcinoma. J Cancer Res Clin Oncol 149, 12923–12929 (2023). https://doi.org/10.1007/s00432-023-05150-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-023-05150-x