Abstract
Purpose
Patients with chronic hepatitis B (CHB) remain at risk for hepatocellular carcinoma during antiviral therapy. We aimed to clarify the values of alpha-fetoprotein (AFP), lectin-reactive fraction of AFP (AFP-L3), and des-γ-carboxyprothrombin (DCP) for early warning of HCC.
Methods
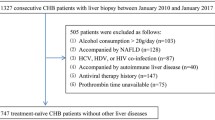
A total of 1348 CHB patients received antiviral therapy and follow-up every 26 weeks. Eighty-four patients with HCC were age-, sex-, and cirrhosis-matched with 168 controls. AFP, AFP-L3, and DCP were compared between the groups from 104 weeks before HCC diagnosis (− 104 w) to the time of diagnosis (0 w).
Results
Of the 84 HCC patients, 60 (71.4%) had early-stage HCC, AFP increased from − 26 w, and AFP-L3 and DCP increased from − 78 w. However, levels were unchanged in controls. ΔAFP, ΔAFP-L3, and ΔDCP showed similar abilities for predicting HCC (P > 0.05). Receiver operating characteristic curve analysis showed that AFP had better diagnostic performance for HCC than AFP-L3, DCP, or their combination. The cut-off values of AFP, AFP-L3, and DCP were 5.3 ng/mL, 1.05%, and 31.5 mAU/mL, respectively. Notably, lower AFP values were required to diagnose HCC in patients with detectable HBV DNA (4.1 ng/mL) or elevated alanine aminotransferase (5.2 ng/mL).
Conclusions
Changes in AFP, AFP-L3, and DCP can help to predict HCC in CHB patients receiving antiviral therapy. A lower AFP value is needed to diagnose HCC, especially in patients with detectable HBV DNA or elevated alanine aminotransferase.




Similar content being viewed by others
Availability of data and material
Reasonable requests can obtain data from the corresponding author.
References
Bertino G, Neri S, Bruno CM et al (2011) Diagnostic and prognostic value of alpha-fetoprotein, des-gamma-carboxy prothrombin and squamous cell carcinoma antigen immunoglobulin M complexes in hepatocellular carcinoma. Minerva Med 102(5):363–371
Bray F, Ferlay J, Soerjomataram I et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424
Buti M, Gane E, Seto WK et al (2016) Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of patients with HBeAg-negative chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol 1(3):196–206
Choi J, Kim GA, Han S et al (2019) Longitudinal assessment of three serum biomarkers to detect very early-stage hepatocellular carcinoma. Hepatology 69(5):1983–1994
Choi J, Kim GA, Han S et al (2020) Earlier alanine aminotransferase normalization during antiviral treatment is independently associated with lower risk of hepatocellular carcinoma in chronic hepatitis B. Am J Gastroenterol 115(3):406–414
European Association for the Study of the Liver (2018) EASL clinical practice guidelines: management of hepatocellular carcinoma. j Hepatol 69(1):182–236
Hsu YC, Yip TC, Ho HJ et al (2018) Development of a scoring system to predict hepatocellular carcinoma in Asians on antivirals for chronic hepatitis B. J Hepatol 69(2):278–285
Jung KS, Kim SU, Song K et al (2015) Validation of hepatitis B virus-related hepatocellular carcinoma prediction models in the era of antiviral therapy. Hepatology 62(6):1757–1766
Kim GA, Seock CH, Park JW et al (2015) Reappraisal of serum alpha-foetoprotein as a surveillance test for hepatocellular carcinoma during entecavir treatment. Liver Int 35(1):232–239
Kim JH, Sinn DH, Kang W et al (2017) Low-level viremia and the increased risk of hepatocellular carcinoma in patients receiving entecavir treatment. Hepatology 66(2):335–343
Kim JH, Kim YD, Lee M et al (2018) Modified PAGE-B score predicts the risk of hepatocellular carcinoma in Asians with chronic hepatitis B on antiviral therapy. J Hepatol 69(5):1066–1073
Kobayashi M, Hosaka T, Ikeda K et al (2011) Highly sensitive AFP-L3% assay is useful for predicting recurrence of hepatocellular carcinoma after curative treatment pre-and postoperatively. Hepatol Res 41(11):1036–1045
Kokudo N, Takemura N, Hasegawa K et al (2019) Clinical practice guidelines for hepatocellular carcinoma: the Japan Society of Hepatology 2017 (4th JSH-HCC guidelines) 2019 update. Hepatol Res 49(10):1109–1113
Lim TS, Kim DY, Han KH et al (2016) Combined use of AFP, PIVKA-II, and AFP-L3 as tumor markers enhances diagnostic accuracy for hepatocellular carcinoma in cirrhotic patients. Scand J Gastroenterol 51(3):344–353
Loglio A, Iavarone M, Vigano M et al (2019) Minimal increases of serum alpha-foetoprotein herald HCC detection in Caucasian HBV cirrhotic patients under long-term oral therapy. Liver Int 39(10):1964–1974
Marrero JA, Kulik LM, Sirlin CB et al (2018) Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 68(2):723–750
Omata M, Cheng AL, Kokudo N et al (2017) Asia–Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int 11(4):317–370
Papatheodoridis GV, Chan HL, Hansen BE et al (2015) Risk of hepatocellular carcinoma in chronic hepatitis B: assessment and modification with current antiviral therapy. J Hepatol 62(4):956–967
Papatheodoridis G, Dalekos G, Sypsa V et al (2016) PAGE-B predicts the risk of developing hepatocellular carcinoma in Caucasians with chronic hepatitis B on 5-year antiviral therapy. J Hepatol 64(6):800–806
Pote N, Cauchy F, Albuquerque M et al (2015) Performance of PIVKA-II for early hepatocellular carcinoma diagnosis and prediction of microvascular invasion. J Hepatol 62(4):848–854
Professional Committee for Prevention and Control of Hepatobiliary and Pancreatic Diseases of Chinese Preventive Medicine Association (2021) Guideline for stratified screening and surveillance of primary liver cancer (2020 Edition). Zhonghua Gan Zang Bing Za Zhi 29(1):25–40
Singhal A, Jayaraman M, Dhanasekaran DN et al (2012) Molecular and serum markers in hepatocellular carcinoma: predictive tools for prognosis and recurrence. Crit Rev Oncol Hematol 82(2):116–140
Wang M, Wang Y, Feng X et al (2017) Contribution of hepatitis B virus and hepatitis C virus to liver cancer in China north areas: experience of the Chinese National Cancer Center. Int J Infect Dis 65:15–21
Wong GL, Chan HL, Tse YK et al (2018) Normal on-treatment ALT during antiviral treatment is associated with a lower risk of hepatic events in patients with chronic hepatitis B. J Hepatol 69(4):793–802
Xu XY, Ding HG, Li WG et al (2019) Chinese guidelines on the management of liver cirrhosis. J Clin Hepatol 35(11):2408–2425
Yang HI, Yeh ML, Wong GL et al (2020) Real-world effectiveness from the Asia Pacific Rim Liver Consortium for HBV risk score for the prediction of hepatocellular carcinoma in chronic hepatitis B patients treated with oral antiviral therapy. J Infect Dis 221(3):389–399
Zhang Y, Liu M, Peng XH et al (2017) Clinical features of patients developing primary hepatocellular carcinoma during anti-HBV therapy with nucleos(t) ide analogues. J Clin Hepatol 33(04):679–683
Acknowledgements
We thank Susan Furness, Ph.D., from Liwen Bianji (Edanz) (www.liwenbianji.cn) for editing the English text of a draft of this manuscript.
Funding
National Science and Technology Major Project (2017ZX10202202, 2013ZX10002004).
Author information
Authors and Affiliations
Contributions
Study design (HM, HY), data collection (QZ, JZ, HL, HW, JR, SS, MY, HY, HM), statistical analysis of data (QZ, SW), manuscript writing (QZ, JZ), serum measurement (MC, KW), critical revision of the manuscript (HM, HY). All authors have made a significant contribution to this study and have approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that no conflict of interest exists concerning this paper.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Medical Ethics Committee of Beijing Friendship Hospital, Capital Medical University (Beijing, China, 2020-P2-194-01) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The requirement for informed consent was waived due to the retrospective nature of the study. Our study was purely observational and not required for clinical trial registration.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, Q., Zhou, J., Li, H. et al. Dynamic changes in three biomarkers predict early-stage hepatocellular carcinoma in patients with chronic hepatitis B receiving antiviral therapy. J Cancer Res Clin Oncol 149, 12691–12701 (2023). https://doi.org/10.1007/s00432-023-05024-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-023-05024-2