Abstract
Purpose
The Prognostic Nutritional Index (PNI), Nutritional Risk Index (NRI), Geriatric Nutritional Risk Index (GNRI), and Controlling Nutritional Status (CONUT) score were devised for quantifying nutritional risk. This study evaluated their properties in detecting compromised nutrition and guiding perioperative management of esophageal cancer patients.
Methods
A prospective institutional database of esophageal cancer patients was reviewed and analyzed. Compromised nutritional status was defined as PNI < 50, NRI < 97.5, GNRI < 92, or CONUT score ≥ 4, respectively. The malnutrition diagnosis consensus established by the European Society of Clinical Nutrition and Metabolism (ESPEN 2015) was selected as reference. Multivariable logistic regression and receiver operating characteristic curve analysis were used. External validation was conducted.
Results
After reviewing the 212-patient database, 192 patients were finally included. Among the four nutritional indexes, the GNRI < 92 showed highest sensitivity (72.0%), specificity (78.9%), and consistency (AUC 0.754, 95% CI 0.672–0.836) with malnutrition diagnosed by ESPEN 2015. The GNRI < 92 showed comparable performance with ESPEN 2015 in recognizing decreased fat mass, fat-free mass, and skeletal muscle mass (all P < 0.01). Both the GNRI < 92 and ESPEN 2015 showed good property in predicting major complications, infectious complications, overall complications and delayed hospital discharge (all P < 0.01), better than PNI < 50, NRI < 97.5, and CONUT score ≥ 4. Regarding the external validation, a retrospective analysis of 155 esophageal cancer patients confirmed the better performance of GNRI < 92 in predicting perioperative morbidities than other 3 nutritional indexes.
Conclusion
The GNRI was optimal in perioperative management of esophageal cancer patients among the four nutritional indexes and was an appropriate alternative to ESPEN 2015 for simplifying nutritional assessment.
Similar content being viewed by others
Introduction
Esophageal cancer is the 12th most common cancer and the seventh most common cause of cancer-related mortality worldwide (Global Burden of Disease Cancer Collaboration 1990). Patients with esophageal cancer naturally suffer from nutritional risk because of metabolic effects and feeding problems. Cancer-related malnutrition is increasingly observed and reported to be associated with dismal prognosis (Baracos 2018). During the past 20 years, malnutrition identification has evolved from the assessment of weight loss, low body mass index (BMI), and reduced food intake to the quantitative measurement of decreased fat-free mass and skeletal muscle mass (Cederholm et al. 2019; Cederholm et al. 2015), contributing to the precise malnutrition diagnosis and corresponding interventions.
Apart from the conventional malnutrition assessment tools, quantitative nutritional indexes were established for quantifying nutritional risk and predicting adverse therapeutic outcomes. These nutritional indexes include the Prognostic Nutritional Index (PNI) (Liao et al. 2019), the Nutritional Risk Index (RNI) (Poulia et al. 2012), the Geriatric Nutritional Risk Index (GNRI) (Bouillanne et al. 2005), and the Controlling Nutritional Status (CONUT) score (Harimoto et al. 2018). All these indexes are based on the routine examination of biochemical and clinical indexes and have the advantage of simplifying nutritional assessment and facilitating dynamic surveillance. During extreme conditions, such as the current novel coronavirus disease (COVID-19) pandemic, conventional malnutrition assessments become less feasible because of difficulties in questionnaire survey and anthropometric measurement. Nutritional indexes are promisingly highlighted in these circumstances. However, no study has compared the efficacy of these nutritional indexes in guiding perioperative management in esophageal cancer patients. Particularly, these nutritional indexes have rarely been validated with the standard malnutrition diagnosis criteria as reference.
In 2015, the European Society of Clinical Nutrition and Metabolism established the first diagnosing consensus on malnutrition (ESPEN 2015) (Cederholm et al. 2015). These criteria consist of three phenotypic criteria (weight loss, low body mass index, and reduced fat-free mass index) and have been validated for diagnosing malnutrition and predicting morbidities and mortalities in patients both in and out of the hospital (Sanchez-Rodriguez et al. 2019; Guerra et al. 2017; Ingadottir et al. 2018).
Above all, this study aimed to investigate the value of the PNI, NRI, GNRI, and CONUT score in detecting compromised nutrition and guiding perioperative management in esophageal cancer patients with the ESPEN 2015 as reference criteria.
Subjects and methods
Study design
We conducted this study by reviewing a prospective database of esophageal cancers established between August 2018 and August 2019 at the Department of Thoracic Surgery, Affiliated Cancer Hospital of Zhengzhou University (Chinese Clinical Trial Registry: 1800017792) (Wang et al. 2020). The study met the ethical standards of the Affiliated Cancer Hospital of Zhengzhou University Ethics Committee (2018127), and all patients signed an informed consent form for the utilization of their data in the prospective database prior to their enrollment. The external validation was conducted by reviewing the prospective database of esophageal cancers at the Department of Thoracic Surgical Oncology, National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College. The consecutive esophageal cancer patients undergoing esophagectomy between June and December 2019 constituted the validation dataset. The external validation was approved by the Research Ethics Committee of Cancer Institute and Hospital of the Chinese Academy of Medical Sciences (2021020718281802).
Patients and data collection
We included patients who met the following criteria: 20–80 years of age, esophageal squamous cell carcinoma, minimally invasive McKeown esophagectomy (McKeown-MIE), and provision of written informed consent. Patients without crucial nutritional data were excluded. Patient biochemical indicators, including the levels of serum albumin and prealbumin, total lymphocyte count, and total cholesterol, were routinely examined and recorded within 3 days before surgery. Body composition parameters, including body weight, fat mass, fat-free mass, and skeletal muscle mass, were automatically measured by bioelectrical impedance analysis (BCA-IB, Tsinghua Tongfang Co., Ltd., Beijing, China) on the morning of the planned surgery (Wang et al. 2020). Indexes of these masses were calculated as masses in kilograms divided by height in square meters (kg/m2). Each patient’s physical status was assessed according to the American Society of Anesthesiologists physical status classification and the Karnofsky performance status (KPS). Evaluation of cardio-pulmonary function was also necessary before surgical approval.
Nutritional assessment
Contents and characteristics of the four nutritional indexes are shown in Supplemental Data 1. PNI is calculated as 10 × serum albumin (g/dl) + 0.005 × total lymphocyte count (per mm3) (Liao et al. 2019). NRI is calculated as follows: NRI = 1.519 × albumin (g/dl) + 41.7 × present weight/usual weight (Poulia et al. 2012). GNRI is calculated according to the following formula: GNRI = 1.489 × albumin (g/dl) + 41.7 × present weight/ideal weight; the ideal weight was calculated using the Lorentz equations (Bouillanne et al. 2005). CONUT score is calculated based on levels of serum albumin, total lymphocyte count, and total cholesterol (Harimoto et al. 2018). All the four indexes screen the nutritional risk as normal, light, moderate, and severe (Supplemental Data 1). Compromised nutritional status is identified as moderate and severe nutritional risk, i.e., PNI < 50, NRI < 97.5, GNRI < 92, and CONUT score ≥ 4, respectively.
ESPEN 2015 diagnoses malnutrition according to one of the following two options (Supplemental Data 1) (Cederholm et al. 2015). Option 1: BMI < 18.5 kg/m2. Option 2: unintentional weight loss > 10% of habitual weight regardless of time or > 5% over 3 months and at least one of the following: a reduced BMI (< 20 kg/m2 or < 22 kg/m2 in subjects younger and older than 70 years, respectively) or a low fat-free mass index (< 15 kg/m2 and < 17 kg/m2 in females and males, respectively).
Treatment strategy
Cancer stages were validated according to the 8th Union for International Cancer Control (UICC)-TNM manuals. Neoadjuvant chemotherapy followed by esophagectomy was the advised strategy for patients with lymph node spread (cN +) or transmural tumor invasion (cT ≥ 3) and KPS ≥ 80%. The neoadjuvant chemotherapy regimen for squamous cell carcinoma consisted of two cycles of paclitaxel administration accompanied by cisplatin (paclitaxel 87.5 mg/m2 on days 1 and 8, cisplatin 25 mg/m2 on days 2–4, repeated every 3 weeks) (Zheng et al. 2019). The NRS 2002 was routinely used to assess the malnutrition risk at per admission. Patients with an NRS score of > 3 points were advised to undertake nutrition support program lasting 7–10 days before surgery (Wang et al. 2020). All included patients underwent McKeown-MIE with a two or three field lymph node dissection; the details of the surgery have been previously described (Zhu et al. 2018; Sun et al. 2018). A “nontube no fasting” early oral feeding program was routinely introduced after surgery. Generally, the patients started oral feeding on postoperative day 1 or 2 without limitations of solid foods and nutrient times, and intravenous nutrition was provided as supplementation to oral feeding and normally stopped on postoperative day 4 or 5 (Wang et al. 2020; Zhu et al. 2018; Sun et al. 2018).
Endpoints
The primary endpoint was the performance of the PNI, NRI, GNRI, and CONUT score in identifying compromised nutritional status compared with the ESPEN 2015. Their properties in recognizing reduced body compositions were particularly concerned. The secondary endpoint was the value of four nutritional indexes in predicting the incidence of perioperative morbidities. Perioperative complications were defined as the complication appearing within 30 days after surgery. We followed the International Consensus on Standardization of Data Collection for Complications Associated with Esophagectomy to identify perioperative complications (Low et al. 2015). Major complications were defined as Clavien-Dindo grade ≥ III (Dindo et al. 2004).
Statistical analysis
Differences between groups were compared with ANOVA, the Mann–Whitney U test, Pearson’s χ2 test, Fisher’s exact test, or the Kruskal–Wallis test based on data characteristics. The sensitivity and specificity values for PNI < 50, NRI < 97.5, GNRI < 92, and CONUT score ≥ 4 with malnutrition identified by ESPEN 2015 were calculated. Cohen’s К statistic and receiver operating characteristic curve analysis were used to assess the diagnostic concordance. Multivariable logistic regression and receiver operating characteristic curve analysis were used to assess the predictive value of compromised nutritional status for perioperative morbidities. Statistic parameters include the odds ratio (OR), the area under the curve (AUC), and the 95% confidence interval (CI). The A two-tailed P value < 0.05 was considered significant. All analyses were conducted using IBM SPSS Statistics for Windows (version 22.0, IBM Corp., Armonk, NY).
Results
During the study period, a total of 238 esophageal cancer patients underwent esophagectomy in our department (Fig. 1). A prospective cohort of 212 patients undergoing McKeown-MIE were established after excluding those undergoing Ivor-Lewis esophagectomy (13 patients) and those without informed consent (3 patients). While reviewing the cohort, 13 patients with adenocarcinoma and 7 patients without crucial nutritional data were excluded, leading to the final inclusion of 192 patients. The average age of was 65.1 ± 7.2 years (Table 1). The mean values of BMI, fat mass index, fat-free mass index, and skeletal muscle mass index were 22.8 ± 2.9 kg/m2, 5.89 ± 1.95 kg/m2, 17.0 ± 1.7 kg/m2, and 8.94 ± 1.33 kg/m2, respectively. The average weight loss within 3 months before surgery was 4.33% (2.37%-7.23%). Preoperative nutritional support was introduced for 88 (43.2%) patients. Seventy-seven (40.1%) patients underwent neoadjuvant chemotherapy followed by McKeown-MIE, while others underwent McKeown-MIE alone. All patients were followed up for at least 3 months, except for 2 (0.9%) patients who died of critical complications within 28 days after surgery.
Nutritional assessments
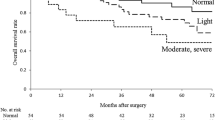
A total of 50 (26.0%) patients were diagnosed with malnutrition according to the ESPEN 2015 (Fig. 2), and the prevalence rates of PNI < 50, NRI < 97.5, GNRI < 92, and CONUT score ≥ 4 were 37.0%, 44.8%, 34.4%, and 33.9%, respectively. The detailed results of the cross-tabulation between ESPEN 2015 and nutritional indexes are provided in Supplemental Data 2. Among the four nutritional indexes, the GNRI < 92 showed the highest sensitivity (72.0%) and specificity (78.9%) and the highest diagnosis agreement (К = 0.461) with malnutrition diagnosed by ESPEN 2015 (Table 2). The receiver operating characteristic curves analyses (Fig. 3) also demonstrated the optimal consistency between GNRI < 92 and malnutrition diagnosed by ESPEN 2015 (AUC 0.754, 95% CI 0.672–0.836).
Clinicopathological characteristics by nutritional status
Patients with compromised nutritional status (regardless of the adopted tool) showed the common characteristics of decreased albumin, prealbumin and total cholesterol (all P < 0.05, Table 1 and Supplemental Data 3). Only the GNRI < 92 and malnutrition diagnosed by the ESPEN 2015 was associated with advanced age and reduced BMI, fat-free mass index, and skeletal muscle mass index (all P < 0.01). The GNRI and ESPEN 2015 also performed better in detecting decreased fat mass index (all P < 0.001) than the PNI, NRI, and CONUT score. Additionally, the NRI < 97.5, GNRI < 92, and malnutrition diagnosed by ESPEN 2015 was commonly associated with increased preoperative weight loss and degenerated carbon monoxide diffusing capacity (all P < 0.001). With respect to pathological parameters, different characteristics were observed according to different indexes.
Perioperative parameters according to nutritional status
Compromised nutritional status was not associated with prolonged operative time (Table 3 and Supplemental Data 4); only the GNRI < 92 was significantly associated with increased estimated blood loss (P = 0.026). Postoperative complications were observed in 86 (44.8%) patients, and the total incidence of major complications was 39 (20.3%). The median postoperative stay was 9.0 (8.0–12.0) days, and delayed hospital discharge was defined as a stay over 9.0 days. The PNI < 50, NRI < 97.5, GNRI < 92, CONUT score ≥ 4, and malnutrition diagnosed by ESPEN 2015 showed the common characteristics of increased incidence of infectious complications, elevated complication grades, and prolonged postoperative hospital stay (all P < 0.05). The GNRI < 92 and malnutrition diagnosed by ESPEN 2015 were specially associated with an increased incidence of cardiac complications, overall complications, and unplanned ICU admission (all P < 0.05).
Values of nutritional indexes for predicting perioperative morbidities
After adjusting for clinicopathological and intraoperative factors, the PNI < 50, NRI < 97.5, GNRI < 92, CONUT score ≥ 4, and malnutrition diagnosed by ESPEN 2015 were commonly predictive of the incidence of major complications and infectious complications (all P < 0.05, Table 4, details were shown in Supplemental Data 5). However, only GNRI < 92 and malnutrition diagnosed by ESPEN 2015 was predictive of the incidence of overall complications and delayed hospital discharge (all P < 0.05). According to the receiver operating characteristic curve analysis (Fig. 4), malnutrition diagnosed by ESPEN 2015 showed the optimal predictive values for postoperative major complications (a), infectious complications (b), overall complications (c), and delayed hospital discharge (d). The GNRI < 92 showed suboptimal values in these regards, although they were observably better than those associated with the PNI, NRI, and CONUT score.
Receiver operating characteristic curves describing the property of nutritional indexes and ESPEN 2015 in predicting the incidence of postoperative morbidities. Major complications (a), infectious complications (b), overall complications (c), and delayed hospital discharge (d). The values of the areas under the curves and 95% confidence intervals are presented
External validation
After reviewing the consecutively 167 patients who underwent McKeown-MIE at the Department of Thoracic Surgical Oncology, National Cancer Center/Cancer Hospital between June and December 2019, 155 patients were finally included to constitute the validation dataset while 12 patients were excluded because of the lacked data. The prevalence rates of PNI < 50, NRI < 97.5, GNRI < 92, and CONUT score ≥ 4 in validation dataset were 40.6%, 41.9%, 28.4%, and 27.1%, respectively (Table 5 and Supplemental Data 6). The GNRI < 92 was still associated with advanced age, lower BMI, and severe weight loss (all P < 0.05). The GNRI < 92 was specially associated with lower levels of baseline albumin, prealbumin, total lymphocyte, and total cholesterol (all P < 0.01). Regarding perioperative morbidities, the GNRI < 92 still perform well in predicting postoperative major complications, infectious complications, overall complications, and delayed hospital discharge in multivariable analyses but with reduced AUCs (Table 6). Although the PNI, NRI, and CONUT score showed good predictive value for infectious complications (all P < 0.05), they performed poorly in predicting other morbidities (Supplemental Data 6).
Discussion
This study is the first to investigate the property of four nutritional indexes, the PNI, NRI, GNRI, and CONUT score, in detecting compromised nutrition status and guiding perioperative management in esophageal cancer patients. Our results demonstrated optimal performance of the GNRI in these regards among the four nutritional indexes and the GNRI is anticipated to substitute the ESPEN 2015 to be used for specific circumstances.
Regarding the identification of compromised nutritional status, the GNRI < 92 showed good sensitivity, specificity and consistency with the ESPEN 2015, better than those of the NRI, PNI and CONUT score. The underlying mechanism could be the adaption of body weight/ideal body weight in GNRI (Bouillanne et al. 2005). Although this parameter cannot describe the precise changes in body compositions, it was proven to macroscopically reflect the decreased fat mass, fat-free mass and skeletal muscle mass; all these were highlighted in the ESPEN 2015 (Cederholm et al. 2015; Bouillanne et al. 2005). The analyses of validation dataset also indicated the good capability of GNRI < 92 in detecting wasted body compositions. Additionally, the GNRI performed better than NRI in identifying malnutrition and tracking reduced body compositions, indicating a higher value of the body weight/ideal body weight than the present weight/usual weight in identifying nutritional risk in esophageal cancer patients (Poulia et al. 2012; Bouillanne et al. 2005). In contrast, the PNI and the CONUT score only include immunonutritional biochemical indicators without any anthropometric measurement (Liao et al. 2019; Harimoto et al. 2018); their poor consistency with the ESPEN 2015 in identifying malnutrition could be reasonable.
The value of the PNI, NRI, GNRI, and CONUT score in perioperative management has not been widely investigated in esophageal cancer patients. A retrospective study by Yamana et al. reported a predictive value of GNRI < 90 for respiratory complications after esophagectomy (Yamana et al. 2015). Yoshida et al. confirmed the association between severe CONUT score and severe morbidities in esophageal cancer patients (Yoshida et al. 2016). In this study, the GNRI < 90 showed better performance in predicting perioperative morbidities than PNI, NRI, and COUNT score, although the performance was inferior to that associated with ESPEN 2015. The external validation also demonstrated the superiority of GNRI in predicting major complications, overall complications, and delayed hospital discharge than other three nutritional indexes. Particularly, although the GNRI and ESPEN 2015 did not include direct immune indicators, they showed good predictive value for infectious complications. These superiorities are believed to originate from the recognition of decreased fat-free mass and skeletal muscle mass which were demonstrated to cause degenerated motor and respiratory function as well as the impaired immune response and metabolic stress (Wang et al. 2020; Nelke et al. 2019; Castaneda et al. 1995). Considering the similar surgical damage and perioperative mortalities between minimally invasive esophagectomy and open esophagectomy (Kalff et al. 2020), the GNRI may also be highlighted in perioperative management of open esophagectomy.
There are inherent differences in methodology and philosophy between four nutritional indexes and conventional malnutrition assessment tools (for example, the ESPEN 2015). These nutritional indexes were devised to classify health-related nutritional risk based on calculations of biochemical indicators and simple clinical indexes; however, the ESPEN 2015 was established to diagnose malnutrition using well-confirmed phenotypic parameters (Cederholm et al. 2015; Liao et al. 2019; Poulia et al. 2012; Bouillanne et al. 2005; Harimoto et al. 2018). The ESPEN 2015 is superior in facilitating the precise diagnosis of malnutrition, while nutritional indexes have the superiority of quantitative assessment and dynamic surveillance of nutritional risk (Sanchez-Rodriguez et al. 2019; Guerra et al. 2017; Ingadottir et al. 2018). It is reasonable that these nutritional indexes failed to reach the efficacy of the ESPEN 2015 in predicting perioperative morbidities in this study. A relief was that the GNRI < 92 showed comparably good performance with ESPEN 2015 in recognizing decreased body compositions and predicting adverse outcomes. Although it seems impossible for nutritional indexes to replace the ESPEN 2015 in routine medical practice, the GNRI is a promising alternative to ESPEN 2015 for nutritional assessment in specific conditions, where conventional assessments are less feasible or unavailable. Notably, with the application of computed tomography-based body composition measurement, the nutritional indexes combined with imaging-based phenotypic assessments could promisingly promote nutritional management for esophageal cancer patients (Hacker et al. 2020).
As of 4 March 2021, a total of 114,653,749 COVID-19 cases have been confirmed worldwide, with 2,550,500 deaths (World Health Organization 2021). With the implementation of social isolation and the recommendations of reducing close contact, nutritional assessment and interventions for esophageal cancer patients become difficult (Rothan and Byrareddy 2020). Based on the mentioned discussion, we regard the GNRI as a promising alternative for traditional malnutrition assessment tools to be used during the pandemic to simplify nutritional assessment and reduce close contact. Particularly, given the susceptibility of cancer patients to COVID-19, anticancer treatments introduced at home or in the community (chemotherapy and targeted therapy) are strongly recommended (You et al. 2020). The GNRI is anticipated to be helpful in nutritional surveillance surrounding these treatments. Clinicians could surveil the GNRI conveniently through telemedicine based on recent biochemical examinations of patients from nearby clinics. The nutritional assessment by GNRI can even be added to the artificial intelligence to optimize medical resources.
The limitations of this study are mainly derived from its nature as a single retrospective cohort analysis. Although the external validation was conducted, the nutritional assessment by ESPEN 2015 was not available. Only Asian patients with esophageal squamous cell carcinoma were included, and the only surgical approach was McKeown-MIE. The benefits of nutritional interventions based on GNRI warrant further investigation.
In conclusion, this study demonstrated the superiority of GNRI in tracing reduced fat-free mass and skeletal muscle mass, identifying compromised nutrition, and predicting perioperative morbidities over the PNI, NRI, and CONUT score. A GNRI < 92 could be highlighted in perioperative management of esophageal cancer patients. Particularly, the GNRI is a promising alternative to ESPEN 2015 to be used in extreme conditions, including the current COVID-19 pandemic.
Data availability
Data described in the manuscript will be made available upon request pending.
Code availability
All the code book and analytic code were provided in the manuscript.
References
Baracos VE (2018) Cancer-associated malnutrition. Eur J Clin Nutr 72(9):1255–1259. https://doi.org/10.1038/s41430-018-0245-4
Bouillanne O, Morineau G, Dupont C et al (2005) Geriatric nutritional risk index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr 82(4):777–783. https://doi.org/10.1093/ajcn/82.4.777
Castaneda C, Charnley JM, Evans WJ et al (1995) Elderly women accommodate to a low-protein diet with losses of body cell mass, muscle function, and immune response. Am J Clin Nutr 62(1):30–39. https://doi.org/10.1093/ajcn/62.1.30
Cederholm T, Bosaeus I, Barazzoni R et al (2015) Diagnostic criteria for malnutrition: an ESPEN consensus statement. Clin Nutr 34:335–340. https://doi.org/10.1016/j.clnu.2015.03.001
Cederholm T, Jensen GL, Correia M et al (2019) GLIM criteria for the diagnosis of malnutrition: a consensus report from the global clinical nutrition community. J Cachexia Sarcopenia Muscle 10(1):207–217. https://doi.org/10.1002/jcsm.12383
Dindo D, Demartines N, Clavien P-A (2004) Classification of surgical complications. Ann Surg 240(2):205–213. https://doi.org/10.1097/01.sla.0000133083.54934.ae
Global burden of disease cancer collaboration (2019) Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the global burden of disease study. JAMA Oncol. https://doi.org/10.1001/jamaoncol.2019.2996
Guerra RS, Fonseca I, Sousa AS et al (2017) ESPEN diagnostic criteria for malnutrition: a validation study in hospitalized patients. Clin Nutr 36(5):1326–1332. https://doi.org/10.1016/j.clnu.2016.08.022
Hacker UT, Hasenclever D, Linder N et al (2020) Prognostic role of body composition parameters in gastric/gastroesophageal junction cancer patients from the EXPAND trial. J Cachexia Sarcopenia Muscle 11:135–144. https://doi.org/10.1002/jcsm.12484
Harimoto N, Yoshizumi T, Inokuchi S et al (2018) Prognostic significance of preoperative controlling nutritional status (CONUT) score in patients undergoing hepatic resection for hepatocellular carcinoma: a multi-institutional study. Ann Surg Oncol 25(11):3316–3323. https://doi.org/10.1245/s10434-018-6672-6
Ingadottir AR, Beck AM, Baldwin C et al (2018) Two components of the new ESPEN diagnostic criteria for malnutrition are independent predictors of lung function in hospitalized patients with chronic obstructive pulmonary disease (COPD). Clin Nutr 37(4):1323–1331. https://doi.org/10.1016/j.clnu.2017.05.031
Kalff MC, Fransen LFC, de Groot EM et al (2020) Long-term survival after minimally invasive versus open esophagectomy for esophageal cancer: a nationwide propensity-score matched analysis. Ann Surg. https://doi.org/10.1097/SLA.0000000000004708 (Publish Ahead of Print)
Liao G, Zhao Z, Yang H et al (2019) Can prognostic nutritional index be a prediction factor in esophageal cancer?: a meta-analysis. Nutr Cancer 72(2):187–193. https://doi.org/10.1080/01635581.2019.1631859
Low DE, Alderson D, Cecconello I et al (2015) International consensus on standardization of data collection for complications associated with esophagectomy: esophagectomy complications consensus group (ECCG). Ann Surg 262(2):286–294. https://doi.org/10.1097/SLA.0000000000001098
Nelke C, Dziewas R, Minnerup J et al (2019) Skeletal muscle as potential central link between sarcopenia and immune senescence. EBioMedicine 49:381–388. https://doi.org/10.1016/j.ebiom.2019.10.034
Poulia KA, Yannakoulia M, Karageorgou D et al (2012) Evaluation of the efficacy of six nutritional screening tools to predict malnutrition in the elderly. Clin Nutr 31(3):378–385. https://doi.org/10.1016/j.clnu.2011.11.017
Rothan HA, Byrareddy SN (2020) The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun 109:102433. https://doi.org/10.1016/j.jaut.2020.102433
Sanchez-Rodriguez D, Marco E, Schott AM et al (2019) Malnutrition according to ESPEN definition predicts long-term mortality in general older population: findings from the EPIDOS study-toulouse cohort. Clin Nutr 38(6):2652–2658. https://doi.org/10.1016/j.clnu.2018.11.016
Sun HB, Li Y, Liu XB et al (2018) Early oral feeding following mckeown minimally invasive esophagectomy: an open-label, randomized, controlled. Noninferiority Trial Ann Surg 267(3):435–442. https://doi.org/10.1097/SLA.0000000000002304
Wang P-Y, Chen X-K, Liu Q et al (2020) Highlighting sarcopeniamanagement forpromoting surgical outcomes in esophageal cancers: evidence from a prospective cohort study. Int J Surg 83:206–215. https://doi.org/10.1016/j.ijsu.2020.09.049
World health organization (2021). Coronavirus disease (COVID-2019) situation reports. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/. Accessed 4 March 2021
Yamana I, Takeno S, Shibata R et al (2015) Is the geriatric nutritional risk index a significant predictor of postoperative complications in patients with esophageal cancer undergoing esophagectomy? Eur Surg Res 55(1–2):35–42. https://doi.org/10.1159/000376610
Yoshida N, Baba Y, Shigaki H et al (2016) Preoperative nutritional assessment by controlling nutritional status (CONUT) is useful to estimate postoperative morbidity after esophagectomy for esophageal cancer. World J Surg 40(8):1910–1917. https://doi.org/10.1007/s00268-016-3549-3
You B, Ravaud A, Canivet A et al (2020) The official French guidelines to protect patients with cancer against SARS-CoV-2 infection. Lancet Oncol 21(5):619–621. https://doi.org/10.1016/s1470-2045(20)30204-7
Zheng Y, Li Y, Liu X et al (2019) Neoadjuvant chemotherapy followed by minimally invasive esophagectomy versus primary surgery for management of esophageal carcinoma: a retrospective study. J Cancer 10(5):1097–1102. https://doi.org/10.7150/jca.29353
Zhu Z, Li Y, Zheng Y et al (2018) Chewing 50 times per bite could help to resume oral feeding on the first postoperative day following minimally invasive oesophagectomy. Eur J Cardiothorac Surg 53(2):325–330. https://doi.org/10.1093/ejcts/ezx291
Acknowledgements
We would like to thank Mantang Qiu and Haoran Li (Department of Thoracic Surgery, Peking University People’s Hospital) for providing help during the research.
Funding
This work was funded by Medical and Health Technology Innovation Project of Chinese Academy of Medical Sciences (grant numbers 2018-12 M-3-003) (Spatial–Temporal Mapping Analysis on Chinese Cancer Burden).
Author information
Authors and Affiliations
Contributions
PW, LX, XL, and YL designed research; PW, XC, QL, LX, and XL conducted research; PW, XC, LX, and RZ analyzed data; PW, XC, QL, and RZ wrote the paper; YL had primary responsibility for final content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, Py., Chen, Xk., Liu, Q. et al. Application of four nutritional risk indexes in perioperative management for esophageal cancer patients. J Cancer Res Clin Oncol 147, 3099–3111 (2021). https://doi.org/10.1007/s00432-021-03585-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-021-03585-8