Abstract
Purpose
Melatonin is an amphipathic indolamine molecule ubiquitously present in all organisms ranging from cyanobacteria to humans. The pineal gland is the site of melatonin synthesis and secretion under the influence of the retinohypothalamic tract. Some extrapineal tissues (skin, lens, gastrointestinal tract, testis, ovary, lymphocytes, and astrocytes) also enable to produce melatonin. Physiologically, melatonin regulates various functions like circadian rhythm, sleep–wake cycle, gonadal activity, redox homeostasis, neuroprotection, immune-modulation, and anticancer effects in the body. Inappropriate melatonin secretion advances the aging process, tumorigenesis, visceral adiposity, etc.
Methods
For the preparation of this review, I had reviewed the literature on the multidimensional activities of melatonin from the NCBI website database PubMed, Springer Nature, Science Direct (Elsevier), Wiley Online ResearchGate, and Google Scholar databases to search relevant articles. Specifically, I focused on the roles and mechanisms of action of melatonin in cancer prevention.
Results
The actions of melatonin are primarily mediated by G-protein coupled MT1 and MT2 receptors; however, several intracellular protein and nuclear receptors can modulate the activity. Normal levels of the melatonin protect the cells from adverse effects including carcinogenesis. Therapeutically, melatonin has chronomedicinal value; it also shows a remarkable anticancer property. The oncostatic action of melatonin is multidimensional, associated with the advancement of apoptosis, the arrest of the cell cycle, inhibition of metastasis, and antioxidant activity.
Conclusion
The present review has emphasized the mechanism of the anti-neoplastic activity of melatonin that increases the possibilities of the new approaches in cancer therapy.
Similar content being viewed by others
Introduction
Melatonin (N-acetyl-5-methoxytryptamine) is a methoxy indolamine compound primarily synthesized and secreted from the pineal gland of humans and other mammals at night in response to dark. This molecule belongs to neurohormone, is derived from serotonin, a specialized product of amino acid tryptophan. The synthesis and secretion of melatonin are regulated by the suprachiasmatic nucleus (SCN) through a complex neural network. The afferent connection arises from melanopsin (very sensitive to blue light) containing intrinsically photosensitive retinal ganglion cells (ipRGCs) which transmits photic signals to SCN via the retinohypothalamic tract (RHT); the efferent connections from SCN innervate the pineal gland through superior cervical ganglia (SCG) as the branch of sympathetic neurons. Exposure of light during day time inhibits melatonin synthesis. Melatonin peak arises during the night and the levels remain low in the day time. At night, darkness induces the liberation of noradrenaline (NA) which advances synthesis and secretion of melatonin. Thus, melatonin is the hormone of the darkness and regulates physiological functions at night. Instead of the pineal gland, extrapineal tissues including the retina, gastrointestinal tract, skin, bone marrow, and lymphocytes also secret melatonin (Tan et al. 2018). Melatonin involves in regulation of different types of physiological functions including circadian rhythm, sleep–wake cycle, reproductive activity, immune-modulation, neuroprotection, gastrointestinal functions, inhibition of carcinogenesis, stem cell proliferation, anti-inflammatory effect, and controlling of aging (Gheban et al. 2019; Zhao et al. 2019).
Melatonin has a prime role in the central circadian clock system. The SCN expresses melatonin receptors on its surfaces. The SCN-melatonin feedback loop regulates the circadian functions. Inappropriate secretion of melatonin causes several health problems such as advancement in the aging process, tumorigenesis and cancer progression, visceral adiposity and cardiovascular disturbances, initiation of type 2 diabetes (Jung-Hynes et al. 2010; Nagorny and Lyssenko 2012; Samanta 2020). Light-at-night alters the pattern of melatonin synthesis and secretion. Song et al. (2016) reported that light-mediated suppression of melatonin was linked with cancer development particularly breast, ovarian, and prostate. The people of the urban area and industrial sectors (night workers and shift workers) are exposed by bright artificial light at night resulting in decrease of blood melatonin levels. These people are prone to the progression of sleep problems, hormonal imbalance, metabolic disorder, neurodegenerative diseases, inflammatory response, and cancer development.
Numerous reports favor the anticancer effects of melatonin. Melatonin acts through the G-protein coupled MT1 and MT2 cell surface receptors; while, another receptor MT3 is the intracellular receptor. The function of MT3-mediated response (membrane receptor-independent) is associated with antioxidant activity (Bondy and Campbell 2018). Moreover, melatonin can bind with intracellular protein (calmodulin, p300, tubulin), transcription factors (NFκB, FOXO3), and nuclear receptors (RZR/RORs, ERα). These binding potentiate the anti-neoplastic activity of melatonin. The actions of melatonin are multidimensional, primarily associated with antioxidant activity, progression of apoptosis, inhibition of proliferation/arrest of the cell cycle, anti-metastasis, and HIF-1α inactivation. Additionally, some reports also indicated that melatonin might be used as an adjuvant with chemotherapeutic agents to improve the potentiality of the treatment. The present review has focused on the mode of action of melatonin and its multiple approaches to cancer prevention including hormone-dependent malignancies. Additionally, this review is also enlightening the possibilities of the use of melatonin in cancer treatment.
Melatonin synthesis and it’s regulation
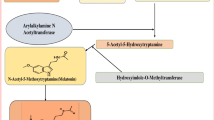
Melatonin is synthesized from an amino acid tryptophan. The human pineal parenchymal cells synthesize melatonin that releases into the blood and the cerebrospinal fluid (CSF). The synthesis of melatonin starts through hydroxylation of tryptophan to 5-hydroxytryptophan by the enzyme tryptophan hydroxylase. Then, 5-hydroxytryptophan is decarboxylated to serotonin. The two key enzymes, arylalkylamine N-acetyl transferase (AANAT) and acetylserotonin O-methyltransferase (ASMT) are responsible for the conversion of serotonin to melatonin (Ostrin 2019) (Fig. 1). Both AANAT and ASMT are primarily expressed in pinealocytes particularly in mitochondria. This process is regulated by NA which is secreted from postganglionic sympathetic nerves that are terminated at the pineal gland (Ostrin 2019; Gheban et al. 2019). NA binds with G-protein coupled β-adrenergic receptor. This binding increases intracellular cAMP (cyclic adenosine monophosphate) levels in pinealocytes followed by activation of protein kinase A II (PKA II) and phosphorylation of cAMP response element binding protein (CREB). Phosphorylated CREB induces the expression of AANAT.
Pinealocytes are specially evolved to synthesize and release melatonin. At the molecular level, the pineal mitochondrial matrix is the site of melatonin synthesis (Suofu et al. 2017). Tan et al. (2016) had reported that the pinealocytes contained large numbers of mitochondria and these mitochondria exhibited dynamic alterations to control melatonin synthesis. The dynamic alterations are mediated by their fission, fusion, and mitophagy activities. At darkness, mitochondrial fusion increases melatonin synthesis to reach the optimum peak; while, mitochondrial fission occurs at day time to reduce melatonin synthesis (Krakowski and Cieciura 1985). Mitochondrial fusion depends on the activity of mitochondrial fusion proteins mitofusin 1 (Mfn1) and Opa1 (Parameyong et al. 2013); however, fission is mediated by dynamin-related protein 1 (DrP1) (Chuang et al. 2016; Ding et al. 2018). Melatonin upregulates the levels of Mfn1 and Opa1 and inhibits the translocation of DrP1. These effects increase mitochondrial fusion and suppress mitochondrial fission for the maximum synthesis of melatonin.
Melatonin is rapidly released from pinealocytes after its synthesis without considerable storage (do Amaral and Cipolla-Neto 2018). It appears within the blood due to the profuse vascularization of the pineal gland. The transport of melatonin through the blood occurs after binding with albumin. Melatonin has a very short half-life. It is rapidly metabolized in the liver by hydroxylation at the C6 of the indole ring to form 6-hydroxymelatonin with the help of cytochrome p450 class of enzyme CYP1A2; then a sulfation reaction converts 6-hydroxymelatonin to 6-sulfatoxy melatonin (aMT6s) for its subsequent urinary excretion.
Melatonin receptors and their functions
The actions of melatonin occur through cell surface receptors. Melatonin has three receptors: MT1, MT2, and MT3 (Liu et al. 2016a; Bahna and Niles 2018; Ostrin 2019). MT1 and MT2 are G-protein coupled membrane receptors. MT1 and MT2 can heterodimerize with G protein receptor (GPR) 50, an orphan G protein-coupled receptor. This GPR50 shows a 45% similarity to human MT1 and MT2 receptors (Levoye et al. 2006). The interaction between MT1 and GPR50 can modify the cellular functions, particularly in neurological activity. The deletion mutation of GPR50 was associated with depressive disorder (Thomson et al. 2005). The third type receptor MT3 (intracellular receptor) is a cytosolic enzyme quinone reductase 2 (QR2, EC1.10.99.2) (Nosjean et al. 2000). This enzyme has potent antioxidant activities. Melatonin binds to the regulatory site of the MT3 and induces its catalytic activity in favor of anti-oxidation. MT3 receptor exerts oncostatic effects even when MT1 and MT2 receptors are blocked by luzindole an antagonist of these two receptors. Inhibition of MT3 receptor by a common inhibitor prazosin shows up-regulation of expression of IL6 and CCL2 genes for angiogenesis and activation of several monokines and chemotactic factors in hepatocellular carcinoma cells (Lin and Chuang 2012).
MT1 receptor activates the inhibitory alpha subunit of heterotrimeric G-protein to decrease cAMP levels followed by the diminution of PKA activity. However, MT2 receptor activates heterotrimeric Gq protein-coupled receptor which activates phospholipase C (PLC) leading to the formation of inositol 1,4,5-trisphosphate (IP3) and 1,2-diacylglycerol. The IP3 increases intracellular Ca2+ levels that activate protein kinase C (PKC). This activation starts downstream actions via MAP kinase and PI3 kinase/Akt pathways and modulates the function of voltage-gated Ca++ channels (Hardeland et al. 2011). The effects of MT1 and MT2 receptors on cancer prevention were studied by using agonists and antagonists. Ramelteon is an agonist of both receptors; while, luzindole acts as an antagonist of these two receptors. The application of ramelteon inhibited proliferation and invasiveness of endometrial cancer cell lines. However, the administration of luzindole suppressed the activity of agonists (Osanai et al. 2017). By using selective inhibitors, numerous studies indicated that the MT1 receptor is more effective to restrict the activity of carcinogenesis in mouse colon cancer cell lines, glioma cell lines, and other caners like oral, breast, and uterine (Bondy and Campbell 2018). Moreover, melatonin increases the synthesis of cyclic 3′,5′-cyclic guanosine monophosphate (cGMP) in the human benign tumor, prostate, ovarian, and other cancer cells. At the downstream steps, cGMP enhances calcium uptake through cyclic nucleotide-gated channels (Rimler et al. 2007). Intracellular calcium activates PKC-α which starts phosphorylation of regulators of G protein signaling (RGS), more specifically RGS10, RGS4. PKC mediated phosphorylation of RGS10 and RGS4 enhances their nuclear trafficking from the cytoplasm. This activity indicates that melatonin receptor differentially affects nuclear-cytoplasmic localization of both Gαi and Gαq specific RGS proteins via PKCα activation (Rimler et al. 2005; Pandi-Perumal et al. 2008). At the nucleus, RGS10 down-regulates the expression of cyclooxygenase-2 (COX-2) and inflammatory cytokines like TNF-α. Inhibition of COX-2 expression decreases prostaglandin E2 (PGE2) synthesis at the cytoplasmic level (Alqinyah et al. 2018).
Melatonin has the ability to cross the cell membrane due to its amphiphilic characteristics. At the intercellular level, melatonin binds with ROR (receptor orphan receptor) and RZR (isoforms as nuclear receptors of retinoic acid receptor superfamily), calmodulin (CaM), calreticulin (calcium-binding protein), tubulin and mitochondrial binding sites. The orphan receptors RZRα, RZRβ, RORα1, RORα2, RORα3, and RORγ form the subfamily within the superfamily of nuclear receptors. RZR/RORs are commonly expressed in the brain and other tissues such as lymphoid organs, gonads, kidneys, gastrointestinal tract, liver, and pancreas (Morgan et al. 1994; Konturek et al. 2007). The orphan receptor RZRβ is mostly expressed in the pineal gland, hypothalamus, and thalamus. The RORγ receptor is commonly found in skeletal muscle thymus, pancreas, prostate, testis, heart, and liver. Expression of RZRα was found in peripheral blood leukocytes (Carlberg and Wiesenberg 1995; Konturek et al. 2007). RZR/RORs have several physiological functions and play a potential role in pathogenesis. RZR/ROR binds at the regulatory regions of many genes and modifies the expression of genes like 5-lipoxygenase, p21WAF1/CIP1, apolipoprotein A-1, N-myc and Purkinje cell protein 2 (Wiesenberg et al. 1998). RORα is essential for the development of the cerebellum, RORα and RORβ are required for maturation of photoreceptors in the retina, and RORγ helps in the development of the secondary lymphoid tissues, including lymph nodes. RORs control the expression of several components of the circadian clock genes and have a potential role in the regulation of various metabolic pathways. Moreover, RORs mediated expressions are associated with several pathological consequences including osteoporosis, several autoimmune diseases, asthma, cancer, and obesity. RZR/ROR, particularly RORα and RORγ have a vital role in inflammation. These two receptors modulate the differentiation and activation of Th1, Th2, Th17, and Treg cells as well as the expression of several cytokines IL-2, IL-4, IL-5, IL-10, IL-13, IL-17, IL-17F, TNF-α, and IFN-γ in a differential manner (Ivanov et al. 2006; Jetten 2009).
RORα is responsible to regulate inflammatory response. Melatonin down-regulates the expression of RORα mediated inflammatory and proliferative factors. The expression of RORα mRNA was down-regulated in breast and lung carcinoma (Zhu et al. 2006; Lu et al. 2007). Alternatively, RORγ promotes differentiation of Th17 cell lineage and influences the expression of IL-2 IL-17, IL-17F, TNF-α, and IFN-γ to increase the inflammation. The animals lacking RORγ (RORγ−/−) showed impaired immune function and exhibited a poor response to inflammation in the OVA-challenged state (Ivanov et al. 2006; Yang et al. 2008a; Jetten 2009). Wang et al. (2016) reported that RORγ was over-expressed in many tumors as the inflammatory response plays a critical role in tumor progression. RORγ regulates the expression of pro-inflammatory cytokines; disturbance in the expression of RORγ promotes inflammatory response as well as cancer progression (Jetten 2009). Melatonin binds with nuclear receptor ROR/RZR to control the transcription signaling of inflammatory cytokines (Wiesenberg et al. 1998; Jockers et al. 2016).
Melatonin inhibits the expression and activity of Sirtuin 1 (SIRT1/Sirt1). SIRT1 is a NAD-dependent deacetylase that regulates the activity of many histone and nonhistone proteins via deacetylation. It modulates the activity of many proteins and transcription factors for the progression of cancer; these include p53, FoxO, PPARγ, NF-κB, p300 (co-activator), and others. Melatonin inactivates the p65 subunit of NF-κB and prevents its translocation to the nucleus. Generally, NF-κB is a family of transcription factors. There are five subunits [NFκB1 (p50), NFκB2 (p52), RelA (p65), RelB, and cRel] in this family. The formation of a homodimer or heterodimer is essential for the activity of NF-κB. Melatonin-induced inactivation of p65 prevents heterodimer formation with p50 resulting inhibition of the expression of genes related to pro-inflammatory cytokines. Thus, melatonin blocks SIRT1 and NF-κB-induced expression of pro-inflammatory cytokines to restrict the exaggeration of the inflammatory response during cancer progression. However, RORα knockout mice are unable to control the inflammatory response, which indicates its role in anti-inflammatory response (García et al. 2015).
An overview of the oncostatic effects of melatonin
The pineal hormone melatonin has potent anticancer effects. Various reports reveal that melatonin has oncostatic effects against carcinogenesis in different tissues including breast, prostate, ovaries, liver, kidney, lung, pancreas, colorectal, skin, and the gastrointestinal system (Li et al. 2017; Gil-Martin et al. 2019). Improper melatonin secretion had been observed in shift workers and night workers as they were exposed by a bright light at night. Brainard et al. (2001) reported that melatonin level decreased in human volunteers those were exposed to monochromatic blue light (464 nm) at night for 90 min (2:00 to 3:30 AM). Straif et al. (2007) also argued that shift work and disruption of melatonin rhythm had carcinogenic effects. Light-at-night increased the risk of health including cancer progression (Schernhammer and Schulmeister 2007; Kantermann and Roenneberg 2009; Song et al. 2016). An epidemiological study suggested that the risk of cancer development in various tissues was positively correlated with long term night shift work (Straif et al. 2007; Hansen 2017). Previously, Pauley (2004) indicated that the occurrence of breast cancer was 36%–60% higher in night shift workers. The rates of breast cancer were high in nurses due to their rotational night shift work. Several studies had established that shift work or light-at-night alters the circadian rhythm and melatonin secretion that are positively associated with the prevalence of breast cancer in the female (Costa et al. 2010; Benabu et al. 2015; Leung et al. 2016; Hansen 2017; James et al. 2017; Kubatka et al. 2018). The occurrence of colorectal cancer was 35% higher in shift workers (Schernhammer et al. 2003). Papantoniou et al. (2015) reported that the rate of prostate cancer in males was high those were present in persistent nighttime illumination.
Several studies have enlightened the anti-cancer effect of melatonin. The oncostatic effects of melatonin are mediated in different ways. The antioxidant property of melatonin protects DNA and protein from oxidative damage. This indolamine prevents uncontrolled cell proliferation by limiting thymidine incorporation, regulates cell cycle, induces apoptosis, and decreases cAMP levels to regulate cellular metabolism (Fig. 2). One of the important examples of the oncostatic effects of melatonin was the study of non-small-cell lung cancer (NSCLC). NSCLC is the 85% cause of lung cancer. The chemotherapeutic treatment of NSCLC is associated with severe toxicity that reduces its therapeutic potentiality. The administration of melatonin solves this problem. Melatonin inhibits proliferation, metastasis, and inflammatory response during NSCLC progression by modulating pre- and pro-apoptotic factors, cytoskeletal remodeling, and NF-κB mediated COX-2 expression (Pourhanifeh et al. 2019). Numerous studies were carried out through in vitro and in vivo system to determine the potency of melatonin in anticancer treatment. The in vitro studies were performed in different cancer cell lines (breast cancer: MCF-7, MDA-MB-231, CMT-U229, Her2.1, and breast cancer-associated fibroblast cells; lung: A549 cells; hepatic: HepG2 and SMMC-7721 cells; gastric: SGC-7901, AGS cells; pancreas: PANC-1, AR42J cells; colorectal; LoVo, HCT 116, HT-29 cells; ovarian: OVCAR-429 and PA-1 cells; prostate: LNCaP, PC3, 22Rm1, DU145, and 22Rv1 cells) to evaluate the doses, effects, and mode of action of melatonin as oncostatic agent (Table 1). Similarly, the in vivo studies were also conducted on different animals (mice: B6C3F1, CB6F1, athymic nude, BALB/c nude; SCID mouse; rat) for the same purpose (see review, Li et al. 2017).
Melatonin synthesis in the pineal gland, the formation of its different derivatives, and the fundamental basis of melatonin mediated anticancer effects. SCG superior cervical ganglion, NA noradrenaline, GPCR G-protein coupled receptor, PKA Protein kinase A, pCREBP phosphorylated cAMP response element binding protein, AANAT arylalkylamine N-acetyl transferase, NAS N-acetyl serotonin, ASMT acetylserotonin O-methyltransferase, CSF cerebro spinal fluid, MT melatonin, 5MTA AFMK: 5-methoxytryptamine, N1-acetyl-N2-formyl 5-methoxykynuramine, AMK N1-acetyl-5-methoxykynuramine, OHM hydroxy melatonin, GSH reduce glutathione, SOD superoxide dismutase, CAT catalase, GPx glutathione peroxidase, CDK cyclin dependent kinase, COX-2 cyclooxygenase2, NF-κB nuclear factor kappa B, EMT epithelial-to-mesenchymal transition, MMP matrix metalloproteinase, HIF-1α hypoxia inducible factor 1 alpha
Mechanism of action of melatonin against cancer development
Activation of apoptotic pathways
Melatonin elicits pleiotropic functions in the body. It acts as a potent apoptotic factor in cancer cells, while modulates anti-apoptotic processes in normal cells. Several studies indicated that melatonin increased apoptosis in hematological cancer, hormone-responsive or unresponsive breast cancer and prostate cancer, hepatic, pancreatic, and colon cancer (Bizzarri et al. 2013). The effect of melatonin on apoptosis was studied on Burkitt lymphoma cells (Trubiani et al. 2005), chronic and acute myeloid leukemia, acute lymphoid leukemia (Casado-Zapico et al. 2011), MCF-7 human breast cancer cells (Cos et al. 2002; Koşar et al. 2016; Gatti et al. 2017), hormone-refractory 22Rv1 human prostate cancer cells (Tam et al. 2007), androgen-dependent LNCaP prostate cancer cells (Joo and Yoo 2009; Kim and Yoo 2010), and human pancreatic carcinoma cells (PANC-1) (Leja-Szpak et al. 2010). Some studies were also conducted on 1,2-dimethylhydrazine induced colon cancer in rats (Anisimov et al. 2000) and N-nitroso-N-methylurea-induced in vivo rat mammary tumor model (Nowfar et al. 2002),
Melatonin can induce both intrinsic and extrinsic pathways of apoptosis (Fig. 3). At the intrinsic apoptotic pathway, melatonin activates caspase-3/8/9, increases cytosolic cytochrome c (cyt c) levels, down-regulates the expression of anti-apoptotic factor Bcl-2 (B cell lymphoma-2), and increases the levels of pro-apoptotic protein Bid (BH3 interacting domain death agonist). Extrinsically, melatonin augmented the expression of Fas and Fas-ligand (Bizzarri et al. 2013). Melatonin treatment in Burkitt lymphoma cells activated caspase-3, increased cytochrome c levels, and preventd the activity of Bcl-2 (Trubiani et al. 2005). A similar mechanism of cell death was also observed in both acute and chronic myeloid leukemia (Bejarano et al. 2009; Rubio et al. 2007). Casado-Zapico et al. (2011) suggested that apoptosis in hematological cancer was mediated by caspase-8 and pro-apoptotic factor Bid in association with Fas, Fas-ligand mediated system.
Primarily, melatonin activates the p53 mediated apoptotic pathway in mammary carcinoma. At early response, melatonin induces the expression of p53, Bax (Bcl-2–associated X protein), p21, and p27 in MCF-7 human breast cancer cells. Additionally, melatonin activates TGFβ-1-induced caspase-7 for late-phase apoptosis. Melatonin downregulates Sirt1 in osteosarcoma; this effect protects p53 from its degradation by increasing the rate of p53 acetylation. The protein p53 triggers the intrinsic apoptotic pathway by elevating Bax and cytochrome c levels, alternatively reduces Bcl-2 levels (Cheng et al. 2013). Melatonin suppresses phosphorylation of Akt and MDM-2 to prevent the inactivation of p53 (Mediavilla et al. 1999). Melatonin increases the p53/MDM2 ratio and downregulates Sirt1. The inhibition of MDM2 and Sirt1, and elevation of p53 activate the apoptotic pathway (Bizzarri et al. 2013). The pharmacological application of melatonin in the MDA-MB-231 cell line (ER-negative breast cancer cells) inhibits COX-2, p300, and NF-κB signaling pathways. Melatonin blocks p300 histone acetyltransferase activity and p300 mediated NF-κB acetylation. These actions stop two functions: (1) NF-κB mediated transcription activation, and (2) p300 induced COX-2 expression followed by prostaglandin synthesis (Wang et al. 2012).
The effect of melatonin in hormone-unresponsive prostate cancer (22Rv1 cells) is MT1 receptor-dependent. Melatonin up-regulates the p27Kip1 gene and protein expression through co-activation of PKC and PKA (protein kinase A) signaling pathways and down-regulates androgen signaling for induction of apoptosis (Tam et al. 2007). An alternative mechanism was also observed in prostate cancer cells. Melatonin action occurs through a receptor-independent system. Melatonin activates cyclic nucleotide-gated channel that leads to increase intracellular calcium levels. Calcium activates protein kinase Cα (PKCα an isoform of PKC) which triggers the extrinsic apoptotic pathway in association with PCKδ (Gonzalez-Guerrico and Kazanietz 2005). Another study indicated that the apoptotic effect of melatonin in colon cancer did not occur through MT1, MT2, and MT3 receptors; melatonin binds with the RZR/ROR receptor followed by induction of p21 factor for apoptosis (Wiesenberg et al. 1998). A different apoptotic pathway was activated in HepG2 liver cancer cells. Melatonin up-regulated FoxO3 mediated pro-apoptotic protein Bim (Carbajo-Pescador et al. 2012); alternatively it decreases the levels of anti-apoptotic protein Bcl-2, inhibits COX-2 expression to intensify endoplasmic reticulum stress-induced apoptosis, and elevated the levels of pro-apoptotic transcription factor CHOP (C/EBP homologous protein) (Zha et al. 2012). All these events promote apoptosis in hepatic cancer cells. Melatonin also activates another apoptotic process in rat hepatoma 7288CTC cells. The application of melatonin decreases cAMP levels that lead to a reduction of phosphorylation of ERK2. This effect diminishes the synthesis of endogenous mitogenic factor 13-hydroxyoctadecadienoic acid (13-HODE) from linoleic acid and also reduces the rate of phosphorylation of MDM-2 and FoxO3a. The dephosphorylated MDM-2 and FoxO3a activate apoptosis (Glasgow et al. 1997; Malmlöf et al. 2007; Yang et al. 2008b). Pancreatic tumor cells are resistant to apoptosis during chemotherapeutic treatment. Administration of melatonin to the PANC-1 cells activates Bcl-2/Bax and caspase-9 proteins for mitochondrial-induced apoptosis without stimulating the caspase 3 mediated DNA fragmentations (Leja-Szpak et al. 2010). Thus, melatonin can adopt different mechanisms of apoptosis which increase the possibilities of its use in cancer treatment.
Chovancova et al. (2017) suggested that calcium might be the responsible factor for the induction of apoptosis in tumor cells. The effect of melatonin was tested on both tumor (ovarian cancer cell line A2780 and colorectal carcinoma cell line DLD1) and non-tumor (endothelial cell line EA.hy926) cells. Melatonin decreases intracellular calcium in tumor cells by activating or expressing type 1 sodium/calcium exchanger (NCX1). It also activates IP3 mediated calcium release from the endoplasmic reticulum (ER) and elevates ER stress. Thus, intracellular calcium depletion or ER stress initiates an apoptotic response. These effects are very less in non-tumor cells. Another important factor is calcium calmodulin-dependent protein kinase II (CaMKII) which is ubiquitously expressed in all cells. The alpha isoform of CaMKII (CaMKIIα) is important for the growth of various cancer including colorectal, prostate, brain, breast, and bone. CaMKIIα regulates the phosphorylation of histone deacetylase 4 (HDAC-4) and controls its nuclear trafficking. Administration of melatonin at pharmacological concentrations promotes the dephosphorylation of HDAC4 by inactivating CaMKIIα. Interaction of melatonin with calmodulin antagonizes Ca2+ binding and blocks CaMKIIα mediated actions (Benitez-King 2006). Dephosphorylation of HDAC4 elevates its nuclear import followed by the induction of apoptosis. The HDAC4 mediated deacetylation of histone 3 at the promoter site of bcl2 (gene of anti-apoptotic protein) reduces bcl-2 expression (Wei et al. 2015). Thus, melatonin has direct effects on the expression of the anti-apoptotic proteins.
Anti-proliferative action
Melatonin has strong anticancer activity. It acts as an anti-proliferative agent and arrests the cell cycle (Fig. 3). The effects of melatonin on different hematological cell lines [Ramos (Epstein–Barr virus-negative BL), SU-DHL-4 (diffuse large B cell lymphoma), DoHH2 (follicular B non-Hodgkin lymphoma) and JURKAT (acute T cell leukemia)] had been studied. The results indicated that the response of melatonin was different in various cell lines. Ramos/DoHH2 showed maximum sensitivity, while the lowest sensitivity observed in the JURKAT cell line. Administration of melatonin arrested the cell cycle of Ramos, DoHH2, and SU-DHL-4 cells in the G1 phase; thus, less numbers of cells were observed in the S phase and G2/M phases (Sánchez-Hidalgo et al. 2012). Melatonin also showed anti-proliferative action in human osteosarcoma cell line MG-63. The cells were halted in the G0/G1 phase without the advancement of the cell cycle towards the next phase (S and G2/M). Melatonin inhibits the expression of G1 phase-related cyclin D1 and CDK4, and G2/M phase-related cyclin B1 and CDK1 (Liu et al. 2013a). Later, Liu et al. (2016b) reported that melatonin inhibited ERK1/2 pathway to arrest the cell cycle in MG-63 osteosarcoma cells; however, melatonin activated ERK1/2 signaling in normal cells for its regular functions (Asghari et al. 2018). Similarly, the cell cycle was also impeded in gastric cancer (GC) cells. The treatment of melatonin in SGC-7901 GC cells revealed that melatonin modulated several cell cycle-related proteins like CDC25A, phospho-CDC25A (at Ser75), p21 and phospho-p21 (at Thr145) (Song et al. 2018). Melatonin was also effective against Hepatocellular carcinoma (HCC) cell lines. Melatonin and sorafenib co-treatment upregulated indigenous cyclin-dependent kinases (CDK)-inhibitor p27 and also decreased the expression of p-AKT, c-myc, cyclin D1, and CDK4/6 protein (Long et al. 2017). A recent study revealed that melatonin arrested the cell cycle in the G2/M phase in both cell culture and in vivo system of murine melanoma model B16-F10 cell line. Pharmacologically, melatonin decreases CDK1 levels and interferes with cytoskeleton organization during cell division. This effect is mediated by diminishing the quantity of cytoskeletal protein beta-actin and alpha-tubulin (Alvarez-Artime et al. 2020). Several studies were performed on human MCF-7 breast cancer cells. Melatonin exerted a cytostatic effect in the G0/G1 phase in MCF-7 cells (Favero et al. 2018). A similar type of cytostatic effect was also observed in T47D and ZR75-1 (estrogen-sensitive) breast cancer cell lines (Cos et al. 1996; Proietti et al. 2013). Melatonin-induced cytostatic effects like inhibition of cell cycle at G1 phase and delayed S phase was observed in other cell lines such as ovarian cancer cells OVCAR-429 (Shen et al. 2016), HepG2 cells of hepatocarcinoma (Martín-Renedo et al. 2008), and SK-MEL-1, MNT-1 cells of melanoma (Cabrera et al. 2010; Kleszczyński et al. 2019).
Additionally, Melatonin inhibits thymidine incorporation during DNA replication in the S phase of the cell cycle to stop the proliferation of cells. The physiological or supraphysiological concentration of melatonin blocked thymidine incorporation in various cancerous cells such as PC3 human prostate tumor cells (Gilad et al. 1999), human androgen-sensitive prostatic tumor cell line-LNCaP (Lupowitz and Zisapel 1999), ovarian adenocarcinoma cell line BG-1 (Petranka et al. 1999), human choriocarcinoma JEG-3 cells (Shiu et al. 2000). Thus, all these above findings indicate the anti-proliferative effects of melatonin.
Oxidative stress (OS), the antioxidant property of melatonin and cancer prevention
Oxidative stress (OS) always creates threatening conditions to maintain the integrity of lipid, protein, and DNA. OS generates highly reactive radicals that start a chain reaction and molecular damage. There are different types of free radicals: (1) reactive oxygen species (ROS: O2–, H2O2 and ·OH), (2) reactive nitrogen species (RNS: ONOO–, NO·, ·NO2), and (3) reactive sulfur species (RSS). The common form of ROS is ·OH (most electrophilic) which can damage lipid, protein, and DNA at its formation site. The ·OH-induced DNA damage occurs vigorously within the cells (Chatgilialoglu et al. 2009). Peroxy radicals (ROO·) are more stable than ·OH and start oxidative damage at any interior part of the cells (Marnett 1987). The alkoxy radicals (RO·) are less toxic than ·OH and ROO·. The toxicity of nitric oxide (NO·) is less, but when it reacts with superoxide radical anion (O·−2 ) produces potent reactive molecule peroxynitrite (ONOO−) (Radi et al. 2001). Generally, RSS is less reactive; more specifically reacts with protein (Abedinzadeh 2001). The redox metals like iron (Fe3+) and copper (Cu2+) play a vital role in oxidative stress within the biological system. These redox metals induce the formation of ·OH within the cell through Fenton-like reaction or the metal-catalyzed Haber–Weiss recombination (MC-HWR) reaction. At the initial stage of the Fenton-like reaction, the metal ions are reduced (Fe3+ to Fe2+ or Cu2+ to Cu+) by O·−2 . The reduced metal ions are reoxidized by H2O2 yielding ·OH (Sies 1997). On the other hand, H2O2 also appears during OS, regulates the signaling of NF-κB, MAP kinase pathways (Srinivas et al. 2019). Oxidative stress and chronic inflammation sustain each other leading to alteration in cytokine levels for augmentation of tumor development and cancerous growth (Basu 2018).
The oxidative damage of DNA is generally exerted by ·OH. The guanosine nucleoside is the most vulnerable to oxidative damage. The oxidation occurs at the site of the guanosine base. The mechanisms of DNA damage are mediated by three processes: (1) hydrogen atom transfer (HAT), (2) radical adduct formation (RAF), and (3) single electron transfer (SET). The deoxyribose moiety of the guanosine nucleoside forms carbon-centered radical cation after deprotonation through SET reaction. The carbon-centered radicals are also formed by HAT in presence of ·OH. Moreover, the imidazole ring of the guanosine molecule produces different adducts through the RAF reaction. The transformed molecules of 2-deoxyguanosine (2dG) are 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxo-dG), 2,6-diamino-4-hydroxy-5-formamidopyrimidine 2′-deoxynucleoside (Fapy-dG) (Galano et al. 2018). The 8-oxo guanine can make G-T or G-A type base pairing; the result is a transition or transverse mutation. Melatonin scavenges ROS to restrict the oxidation of DNA. It also repairs the guanosine radicals and protects DNA from oxidative damage (Tan et al. 2002).
The methylation of the C-5 position of deoxycytidine (dC) is done by an enzyme DNA methyltransferase (DNMT) in the presence of methyl radical (·CH3). The products of carcinogen metabolism such as dimethylhydrazine (DMH), acetaldehyde, tertiary-butyl hydroperoxide (tBuOOH), and cumene hydroperoxide (CuOOH) promote methyl radical formation. Methionine sulfoxide (MetO), alkoxyl radical, acetaldehyde, and dimethyl sulfoxide (DMSO) generate methyl radical either spontaneously or during reaction with ·OH/H2O2/Fe2+ and peroxynitrite. The free radical-mediated dC methylation triggers epigenetic changes in the promoter regions of tumor suppressor genes for the progression of cancer (Kasai et al. 2013). Non-nucleoside compounds [(-)-epigallocatechin-3-gallate (EGCG), hydralazine, procaine, procainamide, RG-108] directly inhibit DNMT. Melatonin and its derivatives (AFMK, AMK, 6OHM, and 4OHM) inhibit DNMT by covering target sequences or by blocking the active site of the enzyme (Korkmaz and Reiter 2008).
OS induces lipid peroxidation form lipid hydroperoxide molecules from polyunsaturated fatty acid (PUFA). The esterified linoleic acid and free linoleic acid produce HPODE [hydroperoxy-(9Z),(11E)-octadecadienoic acid] after ROS-mediated oxidation, which is further reduced to subsequent derivatives of hydroxyoctadecadienoic acids. The ROS-mediated oxidation of arachidonic acid initially gives HPETE (hydroperoxy eicosatetraenoic acid) that is further reduced to (15S)-hydroxy eicosatetraenoic acids (HETE) and (15R)-HETE. Commonly, the lipid peroxidation reaction generates several α,β-unsaturated aldehydic bifunctional electrophiles such as, DDE [trans, trans-2,4-decadienal], EDE [4,5-epoxy-(2E)-decenal], HNE [4-hydroxy-(2E)-nonenal], HPNE [4-hydroperoxy-(2E)-nonenal], MDA [malondialdehyde], ONE [4-oxo-(2E)-nonenal], DODE [9,12-dioxo-(10E)-dodecenoic acid], and 5,8-dioxo-(10E)-octanoic acid. The lipid hydroperoxide-derived bifunctional electrophile molecules react with the bases of DNA to form different adducts like HεdG (heptanone-etheno 2′-deoxyguanosine), εdG (etheno2′-deoxyguanosine), HεdC (heptanone-etheno 2′-deoxycytidine), εdC (etheno 2′-deoxycytidine), HεdA (heptanone-etheno 2′- deoxyadenosine), and εdA (etheno 2′- deoxyadenosine) (Blair 2008). Any types of DNA damage either directly or indirectly promote cancer development.
OS-induced DNA damage plays a vital role in carcinogenesis and chronic inflammation (Murata et al. 2012). DNA damage initiates the development of various cancers in humans; these include breast, prostate, colorectal, gastric, hepatic, lung, and ovarian cancer as well as leukemia, melanoma, and lymphoma (Kryston et al. 2011). DNA damage causes oncogene-induced replication stress which changes the patterns of cell cycles. Oncogenic activity drives continuous cell growth and carcinogenesis. Thus, replication stress ultimately advances genomic instability and tumor development. ROS influences the occurrence of replication stress that alters replication fork speed and promotes the decoupling of DNA polymerase-helicase activity (Srinivas et al. 2019). ROS mediated stimulation activates Myc and Ras signaling to alter the metabolic programs and transformation of normal cells to the oncogenic cells (Maya-Mendoza et al. 2015; Park et al. 2014). Melatonin and its derivatives block Fenton reaction, decreases ·OH, and other free radical generation resulting in diminution of lipid peroxidation and formation of lipid hydroperoxide radical. Thus, melatonin strongly protects the cells from lipid hydroperoxide radical-mediated damages.
According to the mode of action, antioxidants are classified into four groups; (1) Type I antioxidants—chain reaction breaking or radical trapping, (2) Type II antioxidants—preventive, (3) Type III antioxidants—repairing, and (4) Type IV antioxidants- maintenance of intracellular redox homeostasis. Melatonin is a potent antioxidant from its evolutionary period. Melatonin is synthesized from N-acetyl serotonin; while in the reverse reaction, the demethylation of melatonin produces N-acetyl serotonin. Simultaneously, melatonin is also converted to 5-methoxytryptamine (5-MT) through deacetylation and cyclic 3-hydroxymelatonin (c3OHM) after reaction with ·OH. The two derivatives of melatonin N1-acetyl-N2-formyl 5-methoxykynuramine (AFMK), N1-acetyl-5-methoxykynuramine (AMK) are either synthesized via enzymatic reaction or from c3OHM by chemical modification. In the liver, melatonin is metabolized to 6-hydroxy melatonin (6OHM) which is further modified to 4-hydroxy melatonin (4OHM) and 2-hydroxy melatonin (2OHM). The formation of 6OHM, 4OHM, and 2OHM is also possible in the brain and skin. UV radiation can stimulate the transformation of melatonin to 6OHM, 4OHM, and 2OHM (Galano and Reiter 2018) (Fig. 2).
Type I antioxidants directly react with free radicals to generate less toxic molecules. These antioxidants transfer hydrogen atom to the free radicals. The mechanisms of reactions are done in several ways including HAT, SET, RAF, SPLET (sequential proton loss electron transfer), and SEPT (sequential electron proton transfer) (Galano and Reiter 2018). Melatonin has a versatile protective role against free radicals. The potency of melatonin as an antioxidant is very high. It is two times more potent than vitamin E and four times more efficient than ascorbic acid and glutathione for scavenging free radicals (Sofic et al. 2005). Melatonin acts as Type I antioxidant and directly reacts with ·OH (Sofic et al. 2005), RO· (Reiter et al. 2001), ROO· (Galano 2011), and NO· (Siu et al. 2004). These processes are mediated by HAT, RAF, and SET system. Melatonin is quickly quenching the singlet oxygen (1O2) before initiation of further reactions (Matuszak et al. 2003). Thus, melatonin directly scavenges free radicals and protects the cells from their destructive effects (Fig. 3).
Tan et al. (2015) reported that melatonin and its derivatives performed the antioxidant activities in a cascade system which continues the effectiveness of scavenging properties. During the reaction, melatonin forms melatonyl cation radical. Chemically melatonin is composed of an indole ring and two side chains (5-methoxy group and 3-amide group). 5-methoxy group and amino acetyl side chain are present at C5 and C3 positions respectively. The electron-rich indole ring has high resonance stability and electroreactivity. These two properties make the molecule a potent antioxidant. The carbon atoms at the position 2, 3, 4, 6, 7 of the indole moiety are most important for scavenging ·OH or reactive nitrogen radicals. At the reaction time, melatonin is converted to different spices of hydroxy melatonin or cyclic hydroxy melatonin and AFMK. The methoxy group and the acetyl group of the amide react with ·OH to suppress the reactive capacity of hydroxyl radical. The indole ring participates in RAF mediated reaction system; whereas, the 5-methoxy group and amino acetyl side chain involve in HAT type reactions (see review Galano and Reiter 2018). The 5-methoxytryptamine has 50% ability to scavenge ·OH due to a lack of acetyl group in its structure (Tan et al. 2002).
Type II antioxidants act as ·OH inactivating ligand (OIL) (Gaubert et al. 2000). OIL antioxidants must be metal chelating agents. The OIL mediated activities are exerted in two ways (Berthon 1993). The OIL-1 protects the metal from the reaction and prevents the formation of ·OH by blocking the Fenton reaction. Alternatively, OIL-2 forms the metal-OIL-2 complex at the initial stage which gives the chance of formation of ·OH. This nascent ·OH is rapidly trapped by the metal-OIL-2 complex again before the spreading of ·OH within the cellular micro-environment. Melatonin acts as OIL and helps to chelate Cu2+ and Fe3+ (Zatta et al. 2003; Romero et al. 2014). This activity blocks metal ion-induced free radical generation as well as Fenton reaction. Melatonin also inhibits Cu-mediated lipid peroxidation (Parmar et al. 2002).
Type III antioxidants are related to the repairing system of the biomolecules after their oxidative damage rather than scavenging of free radicals. Normally, Type I antioxidants protect the biomolecules against free radical attack. If the protection is not sufficient, then Type III antioxidants repair the biomolecules after damage. The reaction of Type III antioxidants are very similar to HAT and SET reaction system (Galano and Reiter 2018). Melatonin increases the activity of glutathione, vitamin E, and ascorbic acid (Gitto et al. 2001). At the molecular level, melatonin modulates the DNA repair system to manage the OS-induced DNA damage (Majidinia et al. 2017). Numerous reports supported this type of findings. Melatonin influences the activity of genes involved in the DNA repair system via DNA damage responsive pathways (Liu et al. 2013b). Another report indicated that melatonin enhances the expression of DNA repair-related genes after exposure to ionizing radiation (Valizadeh et al. 2017). Ferreira et al. (2013) reported that melatonin protects DNA from cyclophosphamide-induced DNA damage in rats. The DNA damage protective capability of melatonin was also observed in the case of Opisthorchis viverrini-induced oxidative and nitrosative DNA damage in hamster liver (Laothong et al. 2010). Moreover, melatonin involved in phosphorylation of Ser of p53, the classical protein fights against DNA damage (Santoro et al. 2012). All these findings indicated that melatonin always tried to protect DNA from any type of damage.
Type IV antioxidants protect the antioxidant enzymes like catalase (CAT), glutathione peroxidase (GPx), and superoxide dismutase (SOD) by activating the protein nuclear erythroid 2-related factor 2 (NRF2). These antioxidants inhibit pro-oxidative enzymes (xanthineoxidase) and reduce the expression of NF-κB, nitric oxide synthase (NOS), and cyclooxygenase-2 (COX-2). Type IV antioxidants also promote the expression of genes related to DNA repair system. Melatonin maintains the levels of intracellular antioxidant enzymes (Type IV system). This function is mediated by activation of calmodulin which downregulates the activity of the RORα melatonin receptor. This downregulation influences the expression of NF-κB-induced antioxidant enzymes (Tomas-Zapico and Coto-Montes 2005). Fischer et al. (2013) reported that melatonin induced the expression of genes of antioxidant enzymes (CAT, SOD, GPx) in UVR-induced DNA damage.
The other derivatives of melatonin also exhibit antioxidant effects. N-acetylserotonin (NAS) is more effective for scavenging peroxy radicals. It may act as any type of antioxidant (Type I–IV). NAS efficiently protects DNA from H2O2-induced oxidative injuries. 5-methoxytryptamine (5-MT) has the ability to scavenge ·OH. It mainly acts as Type I and Type II antioxidants. Cyclic 3-hydroxymelatonin (c3OHM) is a potent Type I antioxidants and scavenges ·OH. It involves in SET, RAF, and HAT reactions for scavenging free radicals. c3OHM can protect DNA from oxidative damage and formation of 8-OH-dG. It also acts as Type II and Type IV antioxidants. The AFMK prevents DNA damage and lipid peroxidation by scavenging ·OH and H2O2. Type II and Type IV antioxidants activity are also possible for AFMK. AMK is highly reactive against reactive nitrogen species (RNS) and also scavenges ·OH. 6OHM is mostly effective against peroxy radicals. It also acts as Type II and Type IV antioxidants. The antioxidant activity of 4OHM and 2OHM is very similar to 6OHM, but efficiency is less than 6OHM (Galano and Reiter 2018).
Anti-metastatic effects
The growth of cancer cells does not remain restricted in its primary site, cross the basement layer barrier and enter into the circulation where they survive and form cancer in distant tissue (secondary tumorigenesis) (Coghlin and Murray 2010). Initially, the growth of cancer cells starts in a solid tumor; at the subsequent stage formation of the new capillary (angiogenesis) provides oxygen and nutrients to the growing lump. The formation of new vessels opens the route for the migration of tumor cells in the distant past. The cancer cells also reorganize its gene expression. The result is the modulation of transcription and translation of SUMO-specific protease 1, hypoxia inducible factor 1α, and VEGF. Another important fact is the epithelial-to-mesenchymal transition (EMT); this differential change promotes tumor metastasis (Bill and Christofori 2015; Reiter et al. 2017). The basement membrane and extracellular matrix (ECM) are the protective part against cell migration. ECM is the dynamic part and its characteristics vary during cellular activity. Cancer cells release zinc-containing different matrix metalloproteinases (MMP-2 and MMP-9). The gene expression of these enzymes increases during metastasis. The metalloproteinases help to disintegrate the ECM and cells migrate through intravasation, enter in the vascular circulation. Melatonin down-regulates the expression of MMP-2 and MMP-9 genes to reduce the metalloproteinase levels. Before metastasis angiogenesis is a crucial factor. Melatonin suppressed VEGF release in SGC-7901 cells to restrict angiogenesis (Bourboulia and Stetler-Stevenson 2010). Lin et al. (2016) reported that melatonin modulated the activity Akt-Erk/JNK pathways followed by inhibition of NF-κB-mediated MMP-9 transcription and finally prevented metastasis of renal cancer cells.
There are several cell–cell junctional sites; these include tight junctions (TJs), adherens junctions (AJs), gap junctions (GJs), desmosomes, and hemidesmosomes. E-cadherin is the classical component of AJs, which decreases during the early step of metastasis (Hanahan and Weinberg 2011). E-cadherin maintains the integrity of the epithelial sheets. The loss of E-cadherin favors metastasis. Treatment of melatonin in human breast cancer cell line MCF-7 increased the expression of E-cadherin and decreased invasiveness (Cos et al. 1998). Mao et al. (2010) reported that melatonin downregulated the activity of the p38 pathway and inhibited the expression of metalloproteinases-2 and -9 in breast cancer cells. Melatonin modulated the expression of EMT factors like N-cadherin, vimentin, and SNAIL1; while it enhanced E-cadherin and α-catenin levels in hypoxia-induced glioma U251 and SWO-38 cell lines (Chen et al. 2017; Su et al. 2017). A similar type effect was also observed in human ovarian cancer SKOV3 cell line in which melatonin maintained the expression of E-cadherin, but the expression of EMT-related genes SNAIL and vimentin were shutting down (Akbarzadeh et al. 2017). Melatonin up-regulated the tight junctional protein occludin to suppress the migration of human lung adenocarcinoma cell A549 (Zhou et al. 2014). The anti-invasive effect of melatonin was exerted by reducing the expression of αvβ3 integrin in hypoxia-induced glioma cells (Xu et al. 2015). The rearrangement of the cytoskeletal structure is regulated by myosin light-chain kinase (MLCK) and Rho-associated protein kinase (ROCK bears two isoform ROCK1 and ROCK2). Melatonin inhibits microfilament and microtubule organization by modulating the activity of ROCK (Ortiz-Lopez et al. 2009). Moreover, melatonin-induced downregulation of ROCK-1 (Borin et al. 2016) and MLCK (Zhou et al. 2014) showed different regulatory mechanisms. EMT is another factor of cell migration. The signaling pathways such as NF-κB, Wnt, Notch, Hedgehog, AP-1, and growth factor are involved in the EMT process (Su et al. 2017). Melatonin inhibits the expression of NF-κB induced EMT-associated transcription factors like Snail, Slug, Twist, and Zeb (Min et al. 2008). Melatonin activates GSK3β for hindering EMT. Suppression of human epidermal growth factor receptor 2 (HER2)/MAPK/Erk mediated Rsk2 signaling are also possible during melatonin treatment (Mao et al. 2016). The overall effects of melatonin on anti-metastasis are given in Fig. 3.
Effect of melatonin on hypoxia inducible factor-1-alpha (HIF-1α)
Solid tumors comprise approximately 90% of all known cancers which develop from a single mutated cell. Hypoxia is very common in the locally advanced solid tumor that leads to pathophysiological changes within the tumor microenvironment. Hypoxia induces the expression of transcription factor hypoxia-inducible factor-1 alpha (HIF-1α), a key regulator for induction of hundreds of genes those facilitate the adaptation and survival of tumor cells in hypoxic condition (Samanta et al. 2018). HIF-1α regulates the expression of numerous essential genes including IGF2 (insulin like growth factor 2), TGF-α, β3 (transforming growth factor α, β3), VEGF (vascular endothelial growth factor), NOS (nitric oxide synthase), Kir 14 (keratin 14), Kir18 (keratin 18), vimentin, Epo (erythropoietin), heme oxygenase-1, adenylate kinase, transferrin, ceruloplasmin, hexokinase 1, glucose transporter 1 (GLUT1), enolase, glucose-6-phosphate isomerase, phosphofructokinase 1, transglutaminase 2, and leptin for cell proliferation, survival, cytoskeletal reorganization, erythropoiesis, angiogenesis, glucose transport, and metabolism. (Semenza 2010; Masson and Ratcliffe 2014).
Melatonin destabilized HIF-1α levels in the HCT116 human colon cancer cell line (Park et al. 2010). However, another study of Park et al. (2009) indicated that melatonin inhibited the de novo synthesis of HIF-1α by modulating the translational activity of p70S6K (by dephosphorylation) and its target factor RPS6 in DU145, PC-3, and LNCaP prostate cancer cells. The treatment of melatonin in MCF-7 and MDA-MB-231 breast cancer cells showed diminution of expression of both HIF-1α and VEGF-A genes. The protein profiling study indicated that melatonin treatment in hypoxic conditions reduced VEGF-C, VEGF receptors (VEGFR2 and VEGFR3), MMP9, and angiogenin levels (Jardim-Perassi et al. 2016). Similar findings had also been observed in HepG2 hepatocarcinoma cells (Carbajo-Pescador et al. 2013; Colombo et al. 2016).
Angiogenesis is the prime factor for the survival of the tumor, which is primarily influenced by VEGF. Numerous studies indicated that melatonin had a significant inhibitory role in angiogenesis and the expression of VEGF. Melatonin inhibited VEGF expression during in vitro culture of different cell lines such as MCF-7, MDA-MB-231 HepG2, HCT116 DU145, PC-3, LNCaP, human clear cell renal carcinoma cells (ccRCC), RENCA cell line, PANC-1 cells, SCC9 cells, ovarian cancer cells, human gastric cancer cells, villous trophoblast cells, and multiple myeloma cells (see review, Vriend and Reiter 2016).
Various mechanisms had been suggested about melatonin mediated HIF-1α regulation. Translocation of HIF-1α into the nucleus is impeded by melatonin resulting inhibition of binding with p300 and silencing of HIF-1α induced transcriptional activity (Carbajo-Pescador et al. 2013). The von Hippel-Lindau protein (VHL) is an E3 ubiquitin ligase, acts as a tumor suppressor factor (Iwai et al. 1999). The VHL interacts with HIF-1α for its degradation under normoxic conditions (Maxwell et al. 1999). VHL also interacts with RNA polymerase II to regulate the transcription of HIF-1α, and HIF-1α-induced gene expression (Zhang and Yang 2012). In the hypoxic environment, the interaction between VHL and HIF-1α is inhibited due to a lack of hydroxyprolination of HIF-1α. The result is stabilization and translocation of HIF-1α to the nucleus where it heterodimerizes with HIF-1β [an ARNT (aryl hydrocarbon receptor nuclear translocator), consecutively expressed]; the heterodimeric complex binds to hypoxia response element for modulation of transcription of hundreds of genes (Masoud and Li 2015). Although, HIF-1α levels increase during the hypoxic condition, treatment of melatonin increases the binding of HIF-1α to the VHL ligase for its degradation and destabilization (Park et al. 2009). The involvement of the RZR/RORγ receptor and melatonin on HIF-1α levels had studied in SGC-7901 human gastric cancer cells. Normally, indigenous ubiquitin ligase VHL cleaves HIF-1α during the normoxic condition. Another mechanism of HIF-1α degradation is hydroxyproline-independent SUMO-specific pathway. SUMOylation of HIF-1α helps to bind with VHL leading to ubiquitination and proteasomal breakdown. A nuclear protein SUMO-specific protease 1 (SENP1) eliminates SUMO to increase the stability of HIF-1α during hypoxia. Melatonin treatment notably inhibits the expression of RZR/RORγ, SENP1, HIF-1α, and VEGF. The application of melatonin in the RZR/RORγ siRNA transfection system inhibits SENP1 activity as well as HIF-1α levels (Wang et al. 2015).
The HIF-1α is associated with the production of hypoxia-induced ROS in the mitochondria (Brunelle et al. 2005). Mitochondrial oxygen sensing ability induces the complex III of electron transport chain for the production of ROS in a hypoxic environment (Guzy et al. 2005; Guzy and Schumacker 2006). ROS increases the stability of HIF-1α by inactivating the HIF-1α degrading enzyme prolyl-hydroxylase 2 (PHD2) during hypoxia (Guzy et al. 2005, Guzy and Schumacker 2006). Park et al. (2010) observed that melatonin reduced HIF-1α, VEGF, and mitochondrial ROS production in HCT116 colon cancer cells. This finding suggested that the antioxidant effect of melatonin facilitates the ROS reduction capacity followed by activation of PHD2 for VHL ligase mediated degradation of HIF-1α. Thus, melatonin prevents tumor cell survival, metabolism, and angiogenesis in the hypoxic environment by controlling the functions of HIF-1α (Fig. 3).
Effect of melatonin on hormone-dependent cancers
The anticancer effects of melatonin have been studied in almost all types of cancers; however, the studies were initially started on hormone-sensitive mammary tumor models. The oncostatic effects of melatonin are especially emphasized in estrogen-dependent breast and ovarian cancers, and androgen-dependent prostate cancer. A report from the American Cancer Society indicated that about 70% of breast cancers were initially started as a hormone-dependent system where estrogen plays a vital role in tumorigenesis (American Cancer Society 2017). Ovarian cancer (OC) is the prime manifestation of gynecological malignancies. Different steroid hormones estrogens, progesterone, and testosterone are linked with this malignancy. The use of oral contraceptive pills increases the risk of ovarian cancer (Menéndez-Menéndez and Martínez-Campa 2018) and high levels of estradiol also increase the risk of endometrioid cancer (Schock et al. 2014). Prostate cancer (PC) is one of the leading cancers of male, which are mostly androgens (testosterone and dihydrotestosterone) sensitive.
Effect on breast cancer
The nocturnal plasma melatonin concentration decreases in women with estrogen receptor-positive breast cancer indicating that low levels of nighttime melatonin have greater risk in hormone-dependent breast cancer (Tamarkin et al. 1982). Impaired light–dark effect alters the nocturnal plasma melatonin peak. An early study had suggested that exposure of light-at-night to women increases the risk of breast cancer (Stevens 1987). Schernhammer et al. (2001) reported that a higher incidence of breast cancer was profoundly found among the women who worked at least three-night shift duties per month for 20 or more years. Later, James et al. (2017) also reported that outdoor light at night increased the incidence of breast cancer in nurses.
Melatonin and retinoic acid synergistically inhibit the progression of breast cancer. Melatonin has the ability to interact with nuclear receptor binding sites and therefore, can restrict cancer cell proliferation. The effect of melatonin on estrogen-mediated action in breast cancer is exerted by the estrogen receptor α (ERα), not by ERβ (Lai et al. 2008). Hill et al. (2015) reported that melatonin acted through the MT1 receptor in hormone-sensitive [estrogen receptor alpha (ERα)-positive] human breast cancer cells. The expression of ERα is suppressed by melatonin-induced MT1 receptor activity. Binding of melatonin to MT1 receptor lowers intracellular cAMP levels that decreases cAMP-PKA driven CREB mediated prolongs activation of gene expression. Melatonin prevents the binding of estradiol-estrogen receptor α (E-ERα) complex to estrogen response element (ERE) or activator protein-1(AP-1) containing promoters on DNA (Rato et al. 1999). Binding of E-ERα complex to ERE is regulated by intracellular protein calmodulin (CaM). Melatonin is an opponent factor of calmodulin; it changes the structural conformation of calmodulin-ERα leading to inhibition of binding of E-ERα-CaM to ERE. In breast cancer cells, estrogen signaling facilitates the expression of various cancer-promoting factors such as c-Myc, TGFα, and NF-kB. Melatonin critically modulates their expression in cancer cells (Molis et al. 1995).
To control the estrogenic actions in breast cancer, melatonin suppresses estrogen synthesis; it modulates the activity of selective estrogen receptor modulator (SERM) for the alteration of estrogen binding as well as DNA-binding and transcriptional activity. Melatonin inhibits the activity of aromatase, sulfatase, and aldo–keto reductase (AKRs) (González et al. 2007; Dauchy et al. 2014). Melatonin restricts the ERα mediated actions by suppressing the phosphorylation of the estrogen receptor (ERα at S167 and S118), and co-activator (e.g. CaM, SRC-1, CBP/p300, etc.). It also restricts the activity of different kinases (AKT, ERK1/2, PKA, PKC, FAK, GSK3β, c-SRC, etc.) and transcription factors (Ap-1, Elk-1, NF-kB, STAT3, RORα, RAR, RXR, VDR, and PPARγ) (Hill et al. 2011; Dauchy et al. 2014; Hill et al. 2015; Fang et al. 2020). Additionally, melatonin represses the activity of glucocorticoid receptor (GR) in breast cancer cells (Dai et al. 2001), potentiates the transactivation of the vitamin D receptor (VDR) for enhancement of apoptosis (Proietti et al. 2011).
Melatonin shows both cytostatic and cytotoxic activity in breast cancer cells. It involves in inhibition of p38 MAP kinase activity and represses epithelial-mesenchymal transition (EMT); these lead to the suppression of cell proliferation, cell survival, metastasis, and drug resistance capacity. Kiefer et al. (2002) also reported that melatonin exerted suppressive action on estrogen receptor-mediated proliferative activity in MCF-7 human breast cancer cells. It also decreases cAMP levels by inhibiting forskolin-induced and E2-induced activity. Co-administration of 17-β-estradiol (E2) and melatonin or separate application of each compound revealed that melatonin significantly reduced E2-induced ERα transactivation and ERα-ERE binding capacity. Thus, melatonin mediated repression of ERα causes anticancer effects.
Nighttime melatonin is the inhibitor of ovarian steroidogenesis (normal light–dark effect). Similarly, it suppresses estrogen synthesis in breast cancer cell models by inhibiting aromatase, steroid sulfatase (STS), and 17β-hydroxysteroid dehydrogenase type 1 (17β-HSD1) activity. Alternatively, melatonin activates estrogen sulfotransferase (EST) to inactivate estrogen (Martinez-Campa et al. 2009; Blask et al. 2011; Gonzalez–Gonzalez et al. 2018). The pharmacological application of melatonin also inhibits the activity of the aromatase enzyme for the reduction of conversion of androgens to estrogens (Chottanapund et al. 2014). Melatonin also inhibits the expression of aromatase to control the overproduction of estrogen. Treatment of melatonin in MCF-7 breast cancer cells decreases cAMP levels through the MT1 receptor and also represses the expression of aromatase by downregulating the activity of aromatase expressing promoter pII, pI.3, and p1.4. The promoter sites of the aromatase enzyme are regulated by prostaglandin E2 (PGE2). Several reports indicated that COXs (COX-1 and COX-2) were consecutively expressed in breast cancer and prostate cancer cells. High level of COX-2 enzyme enhances PGE2 synthesis which increases intracellular cAMP levels; both PGE2 and cAMP have stimulatory effects on promoters pI.3 and pII to express aromatase enzyme. Another bioactive compound, tetradecanoyl phorbol acetate (TPA) activates PKC for COX activation resulting in enhancement of PGE2 levels. Melatonin acts as a rate-limiting factor in MCF-7 breast cancer cells. It downregulates COX expression and inhibits TPA-induced COX activation to diminish PGE2 synthesis from arachidonic acid. Thus melatonin suppresses aromatase expression by modulating COX-PGE2 activity (Martinez-Campa et al. 2009). Blask and his Co-workers critically studied the impact of nighttime light exposure on carcinogenesis by using human breast cancer xenografts model study. They suggested that melatonin inhibited metabolic activity in the tumor (Warburg effect) by restricting the uptake of free fatty acids, more specifically linoleic acid followed by its conversion to 13-hydroxyoctadecadienoic acid (13-HODE) (Blask et al. 2014). Previously, it was established that 13-HODE had mitogenic activity. It positively regulates the activity of EGF and IGF-1R in human breast cancer xenografts related tumorigenesis (Blask et al. 2005). Thus, night shift work and light-at-night make the women susceptible to breast cancer due to the reduction of blood melatonin levels as well as its anti-estrogenic effects. Melatonin also increases adiponectin secretion, counteracts with elevated concentrations of leptin. The multidimensional effects are potentially beneficial towards the anti-oncogenic activity. Moreover, supplementation of melatonin as an adjuvant also ameliorates the toxicity and side effects of anti-estrogenic drugs commonly used for the treatment of breast cancer (Gonzalez-Gonzalez et al. 2018).
Noncoding microRNAs (miRNAs) normally appear within the cells. They have a crucial role in the regulation of gene expression for the initiation and progression of various human cancers. Melatonin treatment in MCF-7 human breast cancer cells showed that it changed the pattern of miRNAs expression and miRNA-related genes. Melatonin treatment differentially modulated miRNA expression followed by downregulation of target genes related to differentiation, cell growth, cell cycle, and upregulation of genes related to apoptosis, transport, and signal transduction (Lee et al. 2011). Recently, Ferreira et al. (2020) reported that melatonin differentially controled 17 miRNA expressions (11 upregulation and 6 downregulation) to exert anticancer effects in triple-negative breast cancer cells. They also found that melatonin could exhibit anti-tumor activity by controlling proliferation, migration, and c-Myc expression in the same cells even when miRNA expression was suppressed. Global hypomethylation and selective regional hypermethylation are the cause of transcriptional silencing of tumor suppressor genes that leads to the progression of cancer (Esteller 2002; Herman and Baylin 2003). Melatonin treatment in MCF-7 human breast cancer cells suppressed the expression of oncogenic genes, including early growth responsive gene 3 (EGR3), and POU4F2/Brn-3b and it increased the expression of tumor suppressor gene GPC3 (Lee et al. 2013). Glypican-3 (GPC3) is considered as metastasis suppressor; it is the part of proteoglycan that is associated with cell membrane through a GPI anchor. GPC3 regulates migration, proliferation, and cell survival (Peters et al. 2003).
Effect on ovarian cancer
In normal physiological conditions, granulosa cells of preovulatory follicles synthesize ovarian steroid hormone. Melatonin influences progesterone synthesis for ovulation (Tamura et al. 2009). An opposite picture of ovarian steroid (low levels of progesterone and high levels of estradiol) was observed in the postmenopausal period when the action of melatonin decreases due to age-related calcification of pineal gland; this activity increases the risk of endometrial carcinoma (Sandyk et al. 1991). The activity of ovarian granulosa cells is light-independent, thus night-shift work and light-at-night have no direct effect on ovarian cancer (Poole et al. 2011). However, Zhao et al. (2016) reported that a low level of serum melatonin concentration was observed in ovarian cancer patients.
The in vivo animal model study on ovarian cancer had revealed that treatment of melatonin decreased the levels of Her-2, p38, phospho-AKT, and mTOR (Ferreira et al. 2014), inhibited Toll-like receptor 4 (TLR4) mediated MyD88 and TRIF-dependent signaling pathways and reduced NF-κB, TRIF, and IRF-3 levels (Chuffa et al. 2015). On the other hand, melatonin upregulated p53, BAX, and caspase-3 and decreased Bcl-2 levels to promote apoptosis (Chuffa et al. 2016a). The proteomic study showed that long-term application of melatonin regulates metabolism, hypoxia signaling, ER-stress, the stability of proteoglycans, expression of the fatty acid-binding protein (FABP), and mitochondrial heat shock protein 10 (hsp10) (Chuffa et al. 2016b).
The anti-proliferative effect of melatonin was observed in in vitro estrogen-dependent BG-1 ovarian adenocarcinoma cell culture study (Petranka et al. 1999). Melatonin decreased intracellular cAMP levels in ovarian cancer cell lines by regulating MT1 receptor-mediated adenylate cyclase activity. However, melatonin has no significant effect on MT1 receptor expression in cancerous and non-cancerous ovarian cells (Jablonska et al. 2014). Low levels of cAMP modulate the intracellular enzyme activity and also induce the expression of several factors. The ovarian cancer cell lines OVCAR-429 and PA-1 were used in in vitro study. Administration of melatonin down-regulated CDK2 and CDK4 levels and arrested the cell cycle at the G1 phase (Shen et al. 2016). Treatment of melatonin on SK-OV-3 cells inhibited the expression of ZEB1, ZEB2, vimentin to stop the invasion, and also epithelial-mesenchymal transition (Akbarzadeh et al. 2017).
Melatonin increases the efficacy of chemotherapeutic agents. The synergistic effect of melatonin and chemotherapeutic agents (cisplatin) was tested in HTOA cells, OVCAR-3, SK-OV-3 cells; results exhibited the potential anti-proliferate effect, inhibition of ERK phosphorylation along with dephosphorylation of p90RSK and HSP27, and inactivation of phosphatase and tensin homolog (PTEN)/AKT/FOXO3a signaling pathway (Menéndez-Menéndez and Martínez-Campa 2018). Thus melatonin has potential anticancer effects on ovarian carcinoma.
Effect on prostate cancer
The rate of prostate cancer is high among the cancers of male patients. It is associated with shift work and exposure of bright light-at-night (Garcia-Saenz et al. 2018). Application of melatonin in physiological dose on human androgen-sensitive prostatic tumor cell line LNCaP exerts an anti-proliferate effect by capturing the cells in the G0/G1 phase and decreases the number of cells in the S phase (Moretti et al. 2000). A similar result was also observed in human androgen-independent DU 145 prostate cancer cells (Marelli et al. 2000). The pharmacological dose of melatonin gives positive results against cancer progression. Treatment of melatonin in androgen-dependent prostate cancer cells increased p21 levels, decreased NF-κB activation, and Bcl-2 levels; these effects were potentiated by MT1-dependent activity which upregulated the transcription of p27 (Kip1) and inhibited consecutive activation of NF-κB (Shiu et al. 2013). MT1 receptor-mediated action decreases intracellular cAMP levels in association with the down-regulation of metabolic pathways like glycolysis, tricarboxylic acid cycle, and pentose phosphate pathway in androgen-sensitive LNCaP and insensitive PC-3 cells (Hevia et al. 2017). Joo and Yoo (2009) reported that melatonin activated the c-JUN kinase (JNK) and p38 kinase in LNCaP cells for the advancement of apoptosis. The effectiveness of melatonin was also observed in androgen-independent prostate cancer cells where it reduces HIF-1α and VEGF levels (Park et al. 2009). Melatonin administration in human prostate cancer cell lines, as well as in mice with prostate cancer (transgenic adenocarcinoma) showed decrease value of IGF-1, IGFBP3, and other proliferative markers such as PCNA, Ki-67. Melatonin-mediated this suppressive activity was driven by significant inhibition of Sirt1 (NAD+-dependent histone deacetylase) as the cancer cells overexpressed Sirt1 (Jung-Hynes et al. 2011).
Testosterone influences prostate cancer. Melatonin inhibits testosterone production in Leydig cells and restricts prostatic growth. The testosterone biosynthesis in Leydig cells is LH (luteinizing hormone) dependent which increases intracellular cAMP levels. Melatonin binds with MT1 receptor in Leydig cells leading to diminution of cAMP production as well as testosterone synthesis (Frungieri et al. 2005). The results of animal experiments revealed that melatonin acted as a steroidogenic inhibitor, decreased the activity of the steroidogenic acute regulatory protein (StAR), cholesterol side-chain cleavage enzyme (cytochrome p450SCC), and 3β-hydroxysteroid dehydrogenase (3β-HSD) (Frungieri et al. 2005; Mukherjee and Haldar 2014). Moreover, melatonin downregulates GATA-4 and SF-1 transcription factors in Leydig cell lines of mice (Qin et al. 2015). Thus, regulation of testosterone synthesis indirectly exerts oncostatic effects in prostate cancer cells.
Relationship among the circadian clock, melatonin, and anticancer effect
The clock genes are expressed in different tissues including SCN. In humans, 12 core clock genes were identified (Benna et al. 2017). Different clock genes like 3 Period (Per 1, 2, 3), 2 Cryptochrome (Cry1, 2), Brain and muscle aryl hydrocarbon receptor nuclear translocator-like 1 (BMAL1), Circadian locomotor output cycles kaput (CLOCK), 3 Retinoid-related orphan receptor (Ror α, β and γ), 2 Reverse-erythroblastosis (Rev-Erb α and β) and 2 casein kinases 1 (CK1δ and ε) are assembled in a complex regulatory system through positive and negative feedback loops (Pett et al. 2016). Moreover, the products of other genes [Neuronal period-aryl hydrocarbon receptor nuclear translocator single-minded 2 (Npas2/Mop4), Aryl hydrocarbon receptor nuclear translocator like 2 (Arntl2/Mop9), and F-box/LRR-repeat 3 (Fbxl3)] are also involved in clock functions (Siepka et al. 2007).
The Per and Cry genes are rhythmically transcribed which is modulated by CLOCK and BMAL1 complex. CLOCK-BMAL1 complex binds at the upstream of Per, Cry, and 2 orphan nuclear receptors, Ror and Rev-Erb through the specific nucleotide sequence CACGTG, termed as E-box for positive induction of these timekeeping genes (Kwon et al. 2011). Despite CLOCK, NPAS2 (MOP4) can co-ordinately regulate the SCN functions. Actually, NPAS2 is a paralog of CLOCK and enable to heterodimerize with BMAL1 for the regulation of transcriptional activity of different clock genes (Per, Cry), but CLOCK:BMAL1 and NPAS2:BMAL1 differently regulate the clock genes expression at promoter level (De Bruyne et al. 2007). CLOCK-BMAL1 complex induces transcription followed by the translation of clock components (PER, CRY, RORα, REV-ERBα) resulting in the gradual accumulation of these proteins. PER and CRY from heterodimer and then move to the nucleus, where they repress CLOCK-BMAL1 transactivating functions followed by termination of transcription of Per, Cry, Ror and Rev-Erb genes. Thus, the negative feedback loop is completed (Fig. 4) (Gallego and Virshup 2007; Potter 2016). CRY and PER level tends to decrease at night that starts the downfall of repressive activity of CRY-PER complex. This initiates a new cycle of the transcription activation by the CLOCK-BMAL1 complex (Angelousi et al. 2018; Benna et al. 2017). The orphan nuclear receptors RORα and REV-ERBα (transcriptional regulator) have a separate feedback loop in the regulation of BMAL1 mediated expression. RORα and REV-ERBα exert an antagonistic effect. RORα activates Bmal1 transcription while REV-ERBα inhibits transcription. This antagonistic effect maintains the rhythmic level of BMAL1 protein as well as the CLOCK-BMAL1 complex (Gallego and Virshup 2007; Morales-Santana et al. 2019). The degradation of PERs is mediated by cytoplasmic CK1δ and CK1ε, while degradation of CRYs is done by ubiquitin E3 ligase (F-box protein), known as FbxL3.
Mechanism of the positive and negative feedback loop in the regulation of expression core clock genes in the suprachiasmatic nucleus. (+) induction, (−) repression, 3β-HSD 3-beta-hydroxysteroid dehydrogenase, AC acetyl group, BM BMAL1, C CRY, CK1 casein kinase1, ccg clock controlled genes, CL CLOCK, DBP albumin D-site-binding protein, E-box promoter sequence for binding of clock-Bmal1 complex (CACGTG), FBXL3 F-box/LRR repeat protein 3, P PER, Pi phosphate, StAR steroidogenic acute regulatory protein, VEGF vascular endothelial growth factor
The functions of SCN are principally modulated by the light–dark cycle. Another regulatory mechanism is the SCN-melatonin feedback loop. Melatonin reaches to SCN and pars tuberalis (PT) of the pituitary through cerebrospinal fluid (CSF). The SCN expresses melatonin receptors (MT1 and MT2). Moreover, Hablitz et al. (2015) reported that melatonin receptors in SCN were linked with G-protein-coupled inwardly rectifying potassium (GIRK) channels. Binding of melatonin at the surface of SCN regulates the activity of expression of core clock genes (Agez et al. 2007). Melatonin diminishes cAMP levels through the MT1 receptor that might be essential for the regulation of clock genes. Deletion of the MT1 gene, without affecting the MT2 gene reduced the expression of Cry1, Bmal1, and Clock genes (von Gall et al. 2005).
In SCN, melatonin directly interferes with the activity of the ubiquitin-proteosome system. A high level of melatonin at night impedes proteasomal cleavage of BMAL1. This effect increases the availability of BMAL1 and ultimately enhances the levels of CRY, PER, REV-ERBα (Vriend and Reiter 2015). The neuronal activity and gene expression are linked with the melatonin-SCN feedback loop. Mattam and Jagota (2014) reported that exogenous administration of melatonin in rats before the onset of the dark phase significantly increased the transcription activity of Per1, Per2, Cry1, Cry2, and Bmal1. Melatonin could adjust the expression of core clock genes for regulation of cell cycle, survival, and repair mechanisms (de Almeida Chuffa et al. 2019).
Core clock genes make a balance between the expression of oncogenes and tumor suppressor genes. Expressions of core clock genes were deregulated in cancer cells. This disruption influences cell proliferation, migration, and alteration of metabolism in cancer cells (Baan et al. 2009). Moreover, abnormal expression of genes advances the properties of chemoresistance, inhibition of apoptosis, and invasive capacity of cancer cells. Most of the time, the expression of Per1, Per2, Cry1, Cry2, and Bmal1 was decreased in leukemia, melanoma, breast, liver, colon, and prostate cancer. Alternatively, expression of Clock genes might be increased in breast and liver cancer. Another important gene Rev-Erbα was also expressed at a higher rate in breast and colon cancer (see review de Almeida Chuffa et al. 2019). Melatonin had tried to maintain the normal levels of Per, Cry, Bmal1, and RORα in cancer cells. Per and Bmal1 have tumor-suppressive activity.
Furthermore, melatonin blocks SIRT1 dependent cancer promoting activity. The SIRT1 has a crucial role in the regulation of clock genes in cancer cells. SIRT1 is a histone deacetylase protein, involves chromatin remodeling and gene expression. The circadian rhythm dependent NAMPT (NAD synthesizing enzyme) regulates the activity of SIRT1. It plays a vital role in cell proliferation, inhibition of apoptosis, cell survival, etc. The activity of NAMPT is regulated by BMAL1. SIRT1 antagonizes the activity of acetyltransferase resulting in modulation of BMAL1 activity by deacetylation. SIRT1 advances Clock and Bmal1 transcription by controlling the RORα response element. The Clock/Bmal1 complex positively up-regulates the expression of SIRT1 by altering the activity of the E-box promoter. A high level of SIRT1 promotes deacetylation followed by activation of cell proliferation. Melatonin in cancer cells strongly diminishes the CLOCK, BMAL1, and SIRT1 levels by regulating their transcription and exerts anticancer effects (Jung-Hynes et al. 2010; Chang and Guarente 2013; de Almeida Chuffa et al. 2019). Thus, in cancer cells melatonin differentially modulates the expression of clock genes and SIRT1 activity. The pleiotropic effects of melatonin help to regulate the clock genes activity in a separate manner within normal cells and cancer cells.
Promising role of melatonin as an adjuvant in chemotherapy
Cancer cells are mostly resistant to chemotherapy and the efficacy of the curative agents decreases after subsequent uses. Chemotherapeutic agents show undesirable side effects. The combined chemotherapy can overcome the problem of drug resistance capacity of cancer cells (Fic et al., 2017; Pariente et al. 2018; Najafi et al. 2019). Melatonin in combination with chemotherapeutic agents improves several conditions: (1) increases the tolerance capacity of the patients, (2) enhances the efficiency of the drugs, (3) sensitizes the cancer cells towards therapeutic agents, (4) reduces the viability of cancer cells, (5) lowers the toxicity in normal cells and increases their viability, (6) decreases the side effects of the treatment, and (7) enhances the survival rate of the patients (Gao et al. 2017; Najafi et al. 2019). Numerous reports indicated that melatonin could improve the effectiveness of the treatment. 1 mM or 2 mM melatonin was used as an adjuvant with different chemotherapeutic agents (5-fluorouracil, tamoxifen, berberine, cis-diamminedichloroplatinum, cisplatin, proglumide, devazepide, doxorubicin, oxaliplatin, etoposide, rapamycin, aminoglutethimide, irinotecan, gefitinib, thapsigargin, ursolic acid) for in vitro study of various cancers like breast, ovarian, colorectal, NSCLC, and others. The duration of treatment varied from 24 to 48 h (see review Najafi et al. 2019). Adjuvant therapy blocks cell to cell contact, colony formation, and cell viability. This type of treatment increases the effectiveness of the drug by altering IC50 (Yi et al. 2014; Lu et al. 2016; Gao et al. 2017).
Several studies had been performed for evaluation of the potentiality of melatonin as an adjuvant in the regimen of cancer treatment. The studies were conducted on MCF-7 breast cancer cell in combination with doxorubicin (Kosar et al. 2016), lung cancer cells in blending with berberine (Lu et al. 2016), colorectal adenocarcinoma cells in mixture with 5-FU (Pariente et al. 2018), and hepatocellular carcinoma cells in association with cisplatin (Hao et al. 2017). All these experiments had given positive results in favor of melatonin. Chemotherapeutic agent sorafenib is one of the drug choices for the treatment of hepatocellular carcinoma, but there is a chance of drug resistance at the advanced stage of cancer. Melatonin potentiates the cytotoxic effects of sorafenib (tyrosine kinase inhibitor). The combined treatment advances the effect of AKT/p27-mediated arrest of cell growth (Long et al. 2017). Pancreatic ductal adenocarcinoma is adversely resistant to chemotherapeutic agents. The application of sorafenib in combination with melatonin is the potential way for the treatment of pancreatic carcinoma (Fang et al. 2018). Melatonin in association with 5-fluorouracil (5-FU) enhanced the efficacy of the treatment (Lee et al. 2018a; Sakatani et al. 2019). Lee et al. (2018b) reported that melatonin increased the response of chemotherapeutic drug oxaliplatin. Ovarian cancer patients have low levels of plasma melatonin. Ovarian cancer cells show resistance capacity against platinum and taxanes. Melatonin can be used as an adjuvant in the therapeutic regimen of ovarian cancer (Chuffa et al. 2017). Melatonin increases the activity of tamoxifen in MCF-7 breast cancer cells (Wilson et al. 1992).
Two important chemotherapeutic agents (5-FU and cisplatin) are mostly used in the clinical system. 5-FU triggers ROS production for augmentation of apoptosis; cisplatin binds with DNA, forms DNA adduct that influences cellular killing. The colorectal cancer cells are insensitive to the chemotherapeutic agents. Melatonin enhances the activity of 5-FU and cisplatin on colorectal cancer cells by increasing the sensitization effect of the drug. Melatonin also induces cytotoxicity of cisplatin in the different cell lines of ovarian cancer (Pariente et al. 2017). Doxorubicin increases apoptosis through mitochondrial membrane depolarization and caspase activation. Melatonin is the contributor to increasing the efficacy of doxorubicin during the treatment of MCF-7 breast cancer cells (Favero et al. 2018).
A brief report on the clinical trial of melatonin
Several reports are available in favor of the clinical trial of melatonin as an anticancer agent. The applications were done in different types of cancers ranging from head and neck cancer to melanoma (González et al. 2019). The clinical trial revealed that melatonin increased the survival rate of cancer patients. Moreover, it decreased the toxicity and side effects of chemotherapeutic agents and enhanced the tolerance capacity of cancer treatment. Radiotherapy is a common treatment process for head and neck cancer. Severe pain and oral mucositis make difficulties to continue the treatment. The administration of melatonin reduces pain and mucositis. Melatonin supplementation in the clinical trial of the different stages of Hodgkin’s lymphomas exhibited promising results (Todisco et al. 2001; Todisco 2006, 2007). However, contradiction is also available in the field of the clinical trial. Extensive clinical trials were conducted on postmenopausal breast cancer patients (stage 0–III) who had passed a complete anticancer treatment. Oral supplementation of melatonin (3 mg/day for 4 months) did not improve the significant levels of serum biomarkers (estradiol, IGF-1, IGFBP-3) related to breast cancer (Schernhammer et al. 2012). Some outcomes of clinical trials have been given in Table 2.
Pharmacokinetics and safety factors of melatonin
Melatonin is a lipophilic molecule, quickly passes through the membrane and distributed all over the body. The half-life of the molecule is very short (30–57 min). About 90% of body melatonin is metabolized in the liver to form aMT6s (6-sulfatoxymelatonin) (Fig. 1) which excreted through urine. The unmetabolized melatonin is eliminated from the body via bile (Seely et al. 2012).
The acute toxicity of melatonin is very low. The animal study showed that a high dose of melatonin (800 mg/kg body weight) had no lethality. The common symptoms of adverse effects of melatonin are headache, insomnia, rashes, gastric problem, and nightmares (Malhotra et al. 2004). Melatonin is prescribed as medicine for the treatment of sleep disorders and short-term remedy of jetlag. Besag et al. (2019) had tried to search out the adverse effects of melatonin and reported that melatonin treatment slightly increased the day time sleepiness. The chances of other effects like headaches, seep-related problems, dizziness, and hypothermia were below 1%. The safety profile of melatonin is very high as it is non-toxic for the body. Administration of melatonin (1 gm for 30 days) to the human subjects did not show adverse effects (Vijayalaxmi et al. 2002). The doses were used for human trials varied from 10 to 50 mg/day, but there were no serious side effects related to melatonin supplementation (Seely et al. 2012). On the other hand, this indolamine molecule prevents the severe side effects of chemotherapeutic drugs. All these facts indicate the higher safety of melatonin treatment.
Conclusion
Melatonin is a natural indolamine molecule involves in the regulation of normal physiological functions. Inappropriate secretion of melatonin occurs with aging, calcification of pineal gland, exposure of light-at-night, and pathological conditions resulting in sleep disturbance, metabolic disorder, cardiovascular disease, and cancer progression. Melatonin has several pharmacological benefits along with multidimensional anti-anticancer effects. Several studies established its anti-cancer property and also indicated the possibilities of use as an adjuvant with chemotherapeutic drugs. Based on the report from in vitro, in vivo, and clinical trials, it is stated that melatonin has promising approaches in cancer therapy. Moreover, it might be the alternative choice for the treatment of uncontrolled, drug resistance metastatic solid tumors. Unlike other anticancer agents, melatonin has no major aggressive toxicity and has very little chance of post-treatment adverse effects. Finally, it can be concluded that more studies and clinical trials will establish its potentiality as an oncostatic agent and will enlighten the arena of cancer therapy. All these approaches will create new hope to fight against cancer in the near future.
References
Abdel Moneim AE, Guerra-Librero A, Florido J, Shen YQ, Fernández-Gil B, Acuña-Castroviejo D, Escames G (2017) Oral mucositis: melatonin gel an effective new treatment. Int J Mol Sci 18(5):1003. https://doi.org/10.3390/ijms18051003
Abedinzadeh Z (2001) Sulfur-centered reactive intermediates derived from the oxidation of sulfur compounds of biological interest. Can J Physiol Pharmacol 79:166–170
Agez L, Laurent V, Pévet P, Masson-Pévet M, Gauer F (2007) Melatonin affects nuclear orphan receptors mRNA in the rat suprachiasmatic nuclei. Neuroscience 144:522–530. https://doi.org/10.1016/j.neuroscience.2006.09.030
Akbarzadeh M, Movassaghpour AA, Ghanbari H et al (2017) The potential therapeutic effect of melatonin on human ovarian cancer by inhibition of invasion and migration of cancer stem cells. Sci Rep 7(1):1–11 (article 17062)
Alqinyah M, Almutairi F, Wendimu MY, Hooks SB (2018) RGS10 regulates the expression of cyclooxygenase-2 and tumor necrosis factor alpha through a G protein–independent mechanism. Mol Pharmacol 94:1103–1113. https://doi.org/10.1124/mol.118.111674
Alvarez-Artime A, Cernuda-Cernuda R, Naveda F-A, Cepas V, Gonzalez-Menendez P, Fernadez-Vega S, Quiros-Gonzalez I, Sainz RM, Mayo JC (2020) Melatonin-induced cytoskeleton reorganization leads to inhibition of melanoma cancer cell proliferation. Int J Mol Sci 21(2):548. https://doi.org/10.3390/ijms21020548
American Cancer Society (2017) Cancer facts and figures. http://www.cancer.org/research/cancer-factsstatistics/all-cancer-facts-figures/cancer-facts-figures-2017. May 2018
Angelousi A et al (2018) Clock genes alterations and endocrine disorders. Eur J Clinc Investig 48:e12927. https://doi.org/10.1111/eci.12927
Anisimov VN, Popovich IG, Shtylik AV, Zabezhinski MA, Ben Huh H, Gurevich P, Berman V, Tendler Y, Zusman I (2000) Melatonin and colon carcinogenesis III. Effects of melatonin on proliferative activity and apoptosis in colon mucosa and colon tumors induced by 1,2-dimethylhydrazine in rats. Exp Toxicol Pathol 52:71–76. https://doi.org/10.1016/S0940-2993(00)80022-6
Asghari MH, Ghobadi E, Moloudizargari M, Fallah M, Abdollahi M (2018) Does the use of melatonin overcome drug resistance in cancer chemotherapy? Life Sci 196:143–155. https://doi.org/10.1016/j.lfs.2018.01.024
Baan R, Grosse Y, Straif K et al (2009) A review of human carcinogens. Part F: chemical agents and related occupations. Lancet Oncol 10:1143–1144
Bahna SG, Niles LP (2018) Epigenetic regulation of melatonin receptors in neuropsychiatric disorders. Br J Pharmacol 175(16):3209–3219. https://doi.org/10.1111/bph.14058
Basu AK (2018) DNA damage, mutagenesis and cancer. Int J Mol Sci 19:970. https://doi.org/10.3390/ijms19040970
Bejarano I, Redondo PC, Espino J et al (2009) Melatonin induces mitochondrial-mediated apoptosis in human myeloid HL-60 cells. J Pineal Res 46:392–400
Benabu JC, Stoll F, Gonzalez M, Mathelin C (2015) Night work, shift work: breast cancer risk factor? Gynecol Obstet Fertil 43:791–799. https://doi.org/10.1016/j.gyobfe.2015.10.004
Benitez-King G (2006) Melatonin as a cytoskeletal modulator: implications for cell physiology and disease. J Pineal Res 40:1–9
Benna C et al (2017) Genetic variation of clock genes and cancer risk: a field synopsis and meta-analysis. Oncotarget 8:23978–23995. https://doi.org/10.18632/oncotarget.15074
Berthon G (1993) Is copper pro-or anti-inflammatory? A reconciling view and a novel approach for the use of copper in the control of inflammation. Agents Actions 39:210–217
Besag FMC, Vasey MJ, Lao KSJ, Wong ICK (2019) Adverse events associated with melatonin for the treatment of primary or secondary sleep disorders: a systematic review. CNS Drugs 33(12):1167–1186. https://doi.org/10.1007/s40263-019-00680-w
Bill R, Christofori G (2015) The relevance of EMT in breast cancer metastasis: correlation or causality? FEBS Lett 589:1577–1587. https://doi.org/10.1016/j.febslet.2015.05.002
Bizzarri M, Proietti S, Cucina A, Reiter RJ (2013) Molecular mechanisms of the pro-apoptotic actions of melatonin in cancer: a review. Expert Opin Ther Targets 17(12):1483–1496. https://doi.org/10.1517/14728222.2013.834890
Blair IA (2008) DNA adducts with lipid peroxidation products. J Biol Chem 283:15545–15549
Blask DE, Brainard GC, Dauchy RT, Hanifin JP, Davidson LK, Krause JA, Sauer LA, Rivera-Bermudez MA, Dubocovich ML, Jasser SA et al (2005) Melatonin-depleted blood from premenopausal women exposed to light at night stimulates growth of human breast cancer xenografts in nude rats. Cancer Res 65:11174–11184
Blask DE, Hill SM, Dauchy RT, Xiang S, Yuan L, Duplessis T, Mao L, Dauchy E, Sauer LA (2011) Circadian regulation of molecular, dietary, and metabolic signaling mechanisms of human breast cancer growth by the nocturnal melatonin signal and the consequences of its disruption by light at night. J Pineal Res 51:259–269
Blask DE, Dauchy RT, Dauchy EM, Mao L, Hill SM, Greene MW, Belancio VP, Sauer LA, Davidson L (2014) Light exposure at night disrupts host/cancer circadian regulatory dynamics: impact on the Warburg effect, lipid signaling and tumor growth prevention. PLoS One 9:e102776. https://doi.org/10.1371/journal.pone.0102776
Bondy S, Campbell A (2018) Mechanisms underlying tumor suppressive properties of melatonin. Int J Mol Sci 19:E2205. https://doi.org/10.3390/ijms19082205
Borin TF, Arbab AS, Gelaleti GB et al (2016) Melatonin decreases breast cancer metastasis by modulating Rho-associated kinase protein-1 expression. J Pineal Res 60:3–15
Bourboulia D, Stetler-Stevenson WG (2010) Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs): positive and negative regulators in tumor cell adhesion. Semin Cancer Biol 20:161–168. https://doi.org/10.1016/j.semcancer.2010.05.002
Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glickman G, Gerner E et al (2001) Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J Neurosci 21(16):6405–6412
Brunelle JK, Bell EL, Quesada NM, Vercauteren K, Tiranti V, Zeviani M, Scarpulla RC, Chandel NS (2005) Oxygen sensing requires mitochondrial ROS but not oxidative phosphorylation. Cell Metab 1(2005):409–414
Cabrera J, Negrín G, Estévez F, Loro J, Reiter RJ, Quintana J (2010) Melatonin decreases cell proliferation and induces melanogenesis in human melanoma SK-MEL-1 cells. J Pineal Res 49:45–54. https://doi.org/10.1111/j.1600-079X.2010.00765.x
Carbajo-Pescador S, Steinmetz C, Kashyap A et al (2012) Melatonin induces transcriptional regulation of Bim by Fox03a in HepG2 cells. Br J Cancer 108:442–449
Carbajo-Pescador S, Ordonez R, Benet N, Jover R, Garcia-Palomo A, Mauriz JL, Gonzalez-Gallego J (2013) Inhibition of VEGF expression through blockade of Hif1alpha and STAT3 signalling mediates the anti-angiogenic effect of melatonin in HepG2 liver cancer cells. Br J Cancer 109:83–91
Carlberg C, Wiesenberg I (1995) The orphan receptor family RZR/ROR, melatonin and 5-lipoxygenase: An unexpected relationship. J Pineal Res 18:171–178. https://doi.org/10.1111/j.1600-079x.1995.tb00157.x
Casado-Zapico S, Martín V, García-Santos G et al (2011) Regulation of the expression of death receptors and their ligands by melatonin in hematological cancer cell lines and in leukemia cells from patients. J Pineal Res 50:345–355
Chang H-C, Guarente L (2013) SIRT1 mediates central circadian control in the SCN by a mechanism that decays with aging. Cell 153:1448–1460
Chatgilialoglu C, D’Angelantonio M, Guerra M, Kaloudis P, Mulazzani QG (2009) A reevaluation of the ambident reactivity of the guanine moiety towards hydroxyl radicals. Angew Chem Int Ed Engl 48:2214–2217
Chen X, Wang Z, Ma H, Zhang S, Yang H, Wang H et al (2017) Melatonin attenuates hypoxia-induced epithelial-mesenchymal transition and cell aggressive via Smad7/CCL20 in glioma. Oncotarget 8:93580–93592. https://doi.org/10.18632/oncotarget.20525
Cheng Y, Cai L, Jiang P et al (2013) SIRT1 inhibition by melatonin exerts antitumor activity in human osteosarcoma cells. Eur J Pharmacol 715(1–3):219–229. https://doi.org/10.1016/j.ejphar.2013.05.017
Chottanapund S, van Duursen MBM, Navasumrit P et al (2014) Anti-aromatase effect of resveratrol and melatonin on hormonal positive breast cancer cells co-cultured with breast adipose fibroblasts. Toxicol In Vitro 28(7):1215–1221. https://doi.org/10.1016/j.tiv.2014.05.015
Chovancova B, Hudecova S, Lencesova L, Babula P, Rezuchova I, Penesova A, Grman M, Moravcik R, Zeman M, Krizanova O (2017) Melatonin-induced changes in cytosolic calcium might be responsible for apoptosis induction in tumour cells. Cell Physiol Biochem 44:763–777. https://doi.org/10.1159/000485290
Chuang J-I, Pan I-L, Hsieh C-Y, Huang C-Y, Chen P-C, Shin JW (2016) Melatonin prevents the dynamin-related protein 1-dependent mitochondrial fission and oxidative insult in the cortical neurons after 1-methyl-4-phenylpyridinium treatment. J Pineal Res 61:230–240
Chuffa LGA, Fioruci-Fontanelli BA, Mendes LO et al (2015) Melatonin attenuates the TLR4-mediated inflammatory response through MyD88- and TRIF-dependent signaling pathways in an in vivo model of ovarian cancer. BMC Cancer 15(1):34
Chuffa LGA, Alves MS, Martinez M et al (2016a) Apoptosis is triggered by melatonin in an in vivo model of ovarian carcinoma. Endocr Relat Cancer 23(2):65–76
Chuffa LGA, Lupi Júnior LA, Seiva FRF et al (2016b) Quantitative proteomic profiling reveals that diverse metabolic pathways are influenced by melatonin in an in vivo model of ovarian carcinoma. J Proteome Res 15(10):3872–3882
Chuffa LGA, Reiter RJ, Lupi LA (2017) Melatonin as a promising agent to treat ovarian cancer: molecular mechanisms. Carcinogenesis 38:945–952. https://doi.org/10.1093/carcin/bgx054
Coghlin C, Murray GI (2010) Current and emerging concepts in tumour metastasis. J Pathol 222:1–15
Colombo J, Maciel JMW, Ferreira LC, Silva RFD, Zuccari DAPDC (2016) Effects of melatonin on HIF-1α and VEGF expression and on the invasive properties of hepatocarcinoma cells. Oncol Lett 12:231–237. https://doi.org/10.3892/ol.2016.4605
Cos S, Recio J, Sánchez-Barceló EJ (1996) Modulation of the length of the cell cycle time of MCF-7 human breast cancer cells by melatonin. Life Sci 58:811–816. https://doi.org/10.1016/0024-3205(95)02359-3
Cos S, Fernández R, Güézmes A, Sánchez-Barceló EJ (1998) Influence of melatonin on invasive and metastatic properties of MCF-7 human breast cancer cells. Cancer Res 58(19):4383–4390
Cos S, Mediavilla MD, Fernandez R et al (2002) Does melatonin induce apoptosis in MCF-7 human breast cancer cells in vitro? J Pineal Res 32:90–96
Costa G, Haus E, Stevens R (2010) Shift work and cancer—considerations on rationale, mechanisms, and epidemiology. Scand J Work Environ Health 36:163–179. https://doi.org/10.5271/sjweh.2899
Dai J, Ram PT, Yuan L, Spriggs L, Hill SM (2001) Transcriptional repression of RORalpha activity in human breast cancer cells by melatonin. Mol Cell Endocrinol 176:111–120
Dauchy RT, Xiang S, Mao L, Brimer S, Wren MA, Yuan L, Anbalagan M, Hauch A, Frasch T, Rowan BG et al (2014) Circadian and melatonin disruption by exposure to light at night drives intrinsic resistance to tamoxifen therapy in breast cancer. Cancer Res 74:4099–4110
de Almeida Chuffa LG, Seiva FRF, Cucielo MS, Silveira HS, Reiter RJ, Lup LA (2019) Clock genes and the role of melatonin in cancer cells: an overview. Melatonin Res 2(2):133–157. https://doi.org/10.32794/mr11250026
De Bruyne JP, Weaver DR, Reppert SM (2007) CLOCK and NPAS2 have overlapping roles in the suprachiasmatic circadian clock. Nat Neurosci 10(5):543–545. https://doi.org/10.1038/nn1884
Ding M, Ning J, Feng N, Li Z, Liu Z, Wang Y, Wang Y, Li X, Huo C, Jia X et al (2018) Dynamin-related protein 1-mediated mitochondrial fission contributes to post-traumatic cardiac dysfunction in rats and the protective effect of melatonin. J Pineal Res 64:e12447. https://doi.org/10.1111/jpi.12447
do Amaral GF, Cipolla-Neto J (2018) A brief review about melatonin, a pineal hormone. Arch Endocrinol Metab 62(4):472–479. https://doi.org/10.20945/2359-3997000000066
Esteller M (2002) CpG island hypermethylation and tumor suppressor genes: a booming present, a brighter future. Oncogene 21:5427–5440
Fang Z, Jung KH, Yan HH, Kim SJ, Rumman M, Park JH, Han B, Lee JE, Kang YW, Lim JH, Hong SS (2018) Melatonin synergizes with sorafenib to suppress pancreatic cancer via melatonin receptor and PDGFR-β/STAT3 pathway. Cell Physiol Biochem 47:1751–1768
Fang N, Hu C, Sun W, Xu Y, Gu Y, Wu L, Peng Q, Reiter RJ, Liu L (2020) Identification of a novel melatonin-binding nuclear receptor: vitamin D receptor. J Pineal Res 68(1):e12618. https://doi.org/10.1111/jpi.12618
Favero G, Moretti E, Bonomini F, Reiter RJ, Rodella LF, Rezzani R (2018) Promising antineoplastic actions of melatonin. Front Pharmacol 9:1086. https://doi.org/10.3389/fphar.2018.01086
Ferreira SG, Peliciari-Garcia RA, Takahashi-Hyodo SA et al (2013) Effects of melatonin on DNA damage induced by cyclophosphamide in rats. Braz J Med Biol Res 46:278–286
Ferreira GM, Martinez M, Camargo ICC, Domeniconi RF, Martinez FE, Chuffa LGA (2014) Melatonin attenuates Her-2, p38 MAPK, p-AKT, and mTOR levels in ovarian carcinoma of ethanol-preferring rats. J Cancer 5(9):728–735
Ferreira LC, Orso F, Dettori D, Lacerda JZ, Borin TF, Taverna D et al (2020) The role of melatonin on miRNAs modulation in triple-negative breast cancer cells. PLoS One 15(2):e0228062. https://doi.org/10.1371/journal.pone.0228062
Fic M, Gomulkiewicz A, Grzegrzolka J, Podhorska-Okolow M et al (2017) The impact of melatonin on colon cancer cells’ resistance to doxorubicin in an in vitro study. Int J Mol Sci 18(7):1396
Fischer TW, Kleszczyński K, Hardkop LH, Kruse N, Zillikens D (2013) Melatonin enhances antioxidative enzyme gene expression (CAT, GPx, SOD), prevents their UVR-induced depletion, and protects against the formation of DNA damage (8-hydroxy-2′-deoxyguanosine) in ex vivo human skin. J Pineal Res 54:303–312
Frungieri MB, Mayerhofer A, Zitta K, Pignataro OP, Calandra RS, Gonzalez-Calvar SI (2005) Direct effect of melatonin on Syrian hamster testes: melatonin subtype 1a receptors, inhibition of androgen production, and interaction with the local corticotropin-releasing hormone system. Endocrinology 146(3):1541–1552
Galano A (2011) On the direct scavenging activity of melatonin towards hydroxyl and a series of peroxyl radicals. Phys Chem Chem Phys 13:7178–7188
Galano A, Reiter RJ (2018) Melatonin and its metabolites vs oxidative stress: from individual actions to collective protection. J Pineal Res 65:e12514. https://doi.org/10.1111/jpi.12514
Galano A, Tan DX, Reiter RJ (2018) Melatonin: a versatile protector against oxidative DNA damage. Molecules 23:530. https://doi.org/10.3390/molecules23030530
Gallego M, Virshup DM (2007) Post-translational modifications regulate the ticking of the circadian clock. Nat Rev Mol Cell Biol 8:139–148
Gao Y, Xiao X, Zhang C, Yu W, Guo W et al (2017) Melatonin synergizes the chemotherapeutic effect of 5-fluorouracil in colon cancer by suppressing Pi3k/akt and NF-κB/iNOS signaling pathways. J Pineal Res 62(2):e12380
García JA, Volt H, Venegas C, Doerrier C, Escames G, López LC, Acuña-Castroviejo D (2015) Disruption of the NF-κB/NLRP3 connection by melatonin requires retinoid-related orphan receptor-α and blocks the septic response in mice. FASEB J 29:3863–3875
Garcia-Saenz A, Sánchez de Miguel A, Espinosa A et al (2018) Evaluating the association between artificial light-at-night exposure and breast and prostate cancer risk in Spain (MCC-Spain study). Environ Health Perspect 126(4):047011. https://doi.org/10.1289/EHP1837
Gatti G, Lucini V, Dugnani S, Calastretti A, Spadoni G, Bedini A et al (2017) Antiproliferative and pro-apoptotic activity of melatonin analogues on melanoma and breast cancer cells. Oncotarget 8:68338–68353. https://doi.org/10.18632/oncotarget.20124
Gaubert S, Bouchaut M, Brumas V, Berthon G (2000) Copper-ligand interactions and physiological free radical processes. Part 3. Influence of histidine, salicylic acid and anthranilic acid on copper-driven Fenton chemistry in vitro. Free Radic Res 32:451–461
Gheban BA, Rosca IA, Crisan M (2019) The morphological and functional characteristics of the pineal gland. Med Pharm Rep 92(3):226–234. https://doi.org/10.15386/mpr-1235
Gilad E, Laufer M, Matzkin H, Zisapel N (1999) Melatonin receptors in PC3 human prostate tumor cells. J Pineal Res 26:211–220. https://doi.org/10.1111/j.1600-079X.1999.tb00586.x
Gil-Martin et al (2019) The emergence of melatonin in oncology: focus on colorectal cancer. Med Res Rev 9(6):2239–2285. https://doi.org/10.1002/med.21582
Gitto E, Tan DX, Reiter RJ et al (2001) Individual and synergistic antioxidative actions of melatonin: studies with vitamin E, vitamin C, glutathione and desferrrioxamine (desferoxamine) in rat liver homogenates. J Pharm Pharmacol 53:1393–1401
Glasgow WC, Hui R, Everhart AL et al (1997) The linoleic acid metabolite, (13 s)-hydroxyoctadecadienoic acid, augments the epidermal growth factor receptor signaling pathway by attenuation of receptor dephosphorylation. J Biol Chem 272:19269–19276
González A, Martínez-Campa C, Mediavilla MD, Alonso-González C, Sánchez-Mateos S, Hill SM, Sánchez-Barceló EJ, Cos S (2007) Effects of MT1 melatonin receptor overexpression on the aromatase-suppressive effect of melatonin in MCF-7 human breast cancer cells. Oncol Reports 17:947–953
González AG, Revilla NR, Sánchez-Barceló EJ (2019) Clinical uses of melatonin: evaluation of human trials on cancer treatment. Melatonin Res 2(2):47–69. https://doi.org/10.32794/mr11250021
Gonzalez-Gonzalez A, Mediavilla MD, Sanchez-Barcelo EJ (2018) Melatonin: a molecule for reducing breast cancer risk. Molecules 23:E336. https://doi.org/10.3390/molecules23020336
Gonzalez-Guerrico AM, Kazanietz MG (2005) Phorbol ester-induced apoptosis in prostate cancer cells via autocrine activation of the extrinsic apoptotic cascade. J Biol Chem 280:38982–38991
Guzy RD, Schumacker PT (2006) Oxygen sensing by mitochondria at complex III: the paradox of increased reactive oxygen species during hypoxia. Exp Physiol 91:807–819
Guzy RD, Hoyos B, Robin E, Chen H, Liu L, Mansfield KD, Simon MC, Hammerling U, Schumacker PT (2005) Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab 1:401–408
Hablitz LM, Molzof HE, Abrahamsson KE, Cooper JM, Prosser RA, Gamble KL (2015) GIRK channels mediate the nonphotic effects of exogenous melatonin. J Neurosci 35:14957–14965. https://doi.org/10.1523/JNEUROSCI.1597-15.2015
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674
Hansen J (2017) Night shift work and risk of breast cancer. Curr Environ Health Rep 4:325–339. https://doi.org/10.1007/s40572-017-0155-y
Hao J, Li Z, Zhang C, Yu W, Tang Z, Li Y et al (2017) Targeting NF-kB/AP-2b signaling to enhance antitumor activity of cisplatin by melatonin in hepatocellular carcinoma cells. Am JCancer Res 7:13–27
Hardeland R, Cardinali DP, Srinivasan V et al (2011) Melatonin-a pleiotropic, orchestrating regulator molecule. Prog Neurobiol 93:350–384
Herman JG, Baylin SB (2003) Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med 349:2042–2054
Hevia D, Gonzalez-Menendez P, Fernandez-Fernandez M et al (2017) Melatonin decreases glucose metabolism in prostate cancer cells: a 13C stable isotope-resolved metabolomic Study. Int J Mol Sci 18(8):1620. https://doi.org/10.3390/ijms18081620
Hill SM, Blask DE, Xiang S, Yuan L, Mao L, Dauchy RT, Dauchy EM, Frasch T, Duplesis T (2011) Melatonin and associated signaling pathways that control normal breast epithelium and breast cancer. J Mammary Gland Biol Neoplasia 16:235–245
Hill SM, Belancio VP, Dauchy RT, Xiang S, Brimer S, Mao L et al (2015) Melatonin: an inhibitor of breast cancer. Endocr Relat Cancer 22(3):R183–R204. https://doi.org/10.1530/ERC-15-0030
Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelle A, Lafaille JJ, Cua DJ, Littman DR (2006) The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17 + T helper cells. Cell 126:1121–1133
Iwai K, Yamanaka K, Kamura T, Minato N, Conaway RC, Conaway JW, Klausner RD, Pause A (1999) Identification of the von Hippel-lindau tumor-suppressor protein as part of an active E3 ubiquitin ligase complex. Proc Natl Acad Sci USA 96:12436–12441
Jablonska K, Pula B, Zemla A et al (2014) Expression of the MT1 melatonin receptor in ovarian cancer cells. Int J Mol Sci 15(12):23074–23089
James P, Bertrand KA, Hart JE, Schernhammer ES, Tamimi RM, Laden F (2017) Outdoor light at night and breast cancer incidence in the Nurses Health Study II. Environ Health Perspect 125:087010. https://doi.org/10.1289/EHP935
Jardim-Perassi BV, Lourenço MR, Doho GM, Grígolo IH, Gelaleti GB, Ferreira LC, Borin TF, Moschetta MG, Zuccari DAPDC (2016) Melatonin regulates angiogenic factors under hypoxia in breast cancer cell lines. Anti-Cancer Agents Med Chem 16(3):347–358. https://doi.org/10.2174/1871520615666150511094201
Jetten AM (2009) Retinoid-related orphan receptors (RORs): critical roles in development, immunity, circadian rhythm, and cellular metabolism. Nucl Recept Signal 7:e003. https://doi.org/10.1621/nrs.07003
Jockers R, Delagrange P, Dubocovich ML, Markus RP, Renault N, Tosini G et al (2016) Update on melatonin receptors: IUPHAR review 20. Br J Pharmacol 173(18):2702–2725. https://doi.org/10.1111/bph.13536
Joo SS, Yoo YM (2009) Melatonin induces apoptotic death in LNCaP cells via p38 and JNK pathways: therapeutic implications for prostate cancer. J Pineal Res 47:8–14
Jung-Hynes B, Reiter RJ, Ahmad N (2010) Sirtuins, melatonin and circadian rhythms: building a bridge between aging and cancer. J Pineal Res 48:9–19. https://doi.org/10.1111/j.1600-079X.2009.00729.x
Jung-Hynes B, Schmit TL, Reagan-Shaw SR, Isiddiqui IA, Mukhtar H, Ahmad N (2011) Melatonin, a novel Sirt1 inhibitor, imparts antiproliferative effects against prostate cancer in vitro in culture and in vivo in TRAMP model. J Pineal Res 50(2):140–149
Kantermann T, Roenneberg T (2009) Is light-at-night a health risk factor or a health risk predictor? Chronobiol Int 2:1069–1074. https://doi.org/10.3109/07420520903223984
Kasai H, Kawai K, Li Y-S (2013) Free radical-mediated cytosine C-5 methylation triggers epigenetic changes during Carcinogenesis. BioMol Concepts 4(3):213–220. https://doi.org/10.1515/bmc-2012-0052
Kiefer T, Ram PT, Yuan L, Hill SM (2002) Melatonin inhibits estrogen receptor transactivation and cAMP levels in breast cancer cells. Breast Cancer Res Treat 71:37–45. https://doi.org/10.1023/A:1013301408464
Kim CH, Yoo YM (2010) Melatonin induces apoptotic cell death via p53 in LNCaP cells. Korean J Physiol Pharmacol 14:365–369
Kleszczyński K, Kim TK, Bilska B, Sarna M, Mokrzynski K, Stegemann A, Pyza E, Reiter RJ, Steinbrink K, Böhm M, Slominski AT (2019) Melatonin exerts oncostatic capacity and decreases melanogenesis in human MNT-1 melanoma cells. J Pineal Res 67(4):e12610. https://doi.org/10.1111/jpi.12610
Konturek SJ, Konturek PC, Brzozowska I et al (2007) Localization and biological activities of melatonin in intact and diseased gastrointestinal tract (GIT). J Physiol Pharmacol 58:381–405
Korkmaz A, Reiter RJ (2008) Epigenetic regulation: a new research area for melatonin? J Pineal Res 44:41–44. https://doi.org/10.1111/j.1600-079X.2007.00509.x
Koşar PA, Naziroğlu M, Övey ÝS, Çiğ B (2016) Synergic effects of doxorubicin and melatonin on apoptosis and mitochondrial oxidative stress in MCF-7 breast cancer cells: involvement of TRPV1 channels. J Membr Biol 249:129–140. https://doi.org/10.1007/s00232-015-9855-0
Krakowski G, Cieciura L (1985) Ultrastructural studies on the pinealocyte mitochondria during the daytime and at night. J Pineal Res 2:315–324
Kryston TB, Georgiev AB, Pissis P, Georgakilas AG (2011) Role of oxidative stress and DNA damage in human carcinogenesis. Mutat Res 711:193–201. https://doi.org/10.1016/j.mrfmmm.2010.12.016
Kubatka P, Zubor P, Busselberg D, Kwon TK, Adamek M, Petrovic D, Opatrilova R, Gazdikova K, Caprnda M, Rodrigo L et al (2018) Melatonin and breast cancer: Evidences from preclinical and human studies. Crit Rev Oncol Hematol 122:133–143. https://doi.org/10.1016/j.critrevonc.2017.12.018
Kwon I et al (2011) Mammalian molecular clocks. Exp Neurobiol 20:18–28. https://doi.org/10.5607/en.2011.20.1.18
Lai L, Yuan L, Chen Q, Dong C, Mao L, Rowan B, Frasch T, Hill SM (2008) The Galpha i and Galpha q proteins mediate the effects of melatonin on steroid/thyroid hormone receptor transcriptional activity and breast cancer cell proliferation. J Pineal Res 45:476–488
Laothong U, Pinlaor P, Hiraku Y et al (2010) Protective effect of melatonin against Opisthorchis viverrini-induced oxidative and nitrosative DNA damage and liver injury in hamsters. J Pineal Res 49:271–282
Lee SE, Kim SJ, Youn JP, Hwang SY, Park CS, Park YS (2011) MicroRNA and gene expression analysis of melatonin-exposed human breast cancer cell lines indicating involvement of the anticancer effect. J Pineal Res 51:345–352. https://doi.org/10.1111/j.1600-079X.2011.00896.x
Lee SE, Kim SJ, Yoon HJ, Yu SY, Yang H, Jeong SI, Hwang SY, Park CS, Park YS (2013) Genome-wide profiling in melatonin-exposed human breast cancer cell lines identifies differentially methylated genes involved in the anticancer effect of melatonin. J Pineal Res 54:80–88. https://doi.org/10.1111/j.1600-079X.2012.01027.x
Lee JH, Yoon YM, Han YS, Yun CW, Lee SH (2018a) Melatonin promotes apoptosis of oxaliplatin-resistant colorectal cancer cells through inhibition of cellular prion protein. Anticancer Res 38:1993–2000
Lee JH, Yun CW, Han YS, Kim S, Jeong D, Kwon HY, Kim H, Baek MJ, Lee SH (2018b) Melatonin and 5-fluorouracil co-suppress colon cancer stem cells by regula-ting cellular prion protein-Oct4 axis. J Pineal Res 65:e12519. https://doi.org/10.1111/jpi.12519
Leja-Szpak A, Jaworek J, Pierzchalski P, Reiter RJ (2010) Melatonin induces pro-apoptotic signaling pathway in human pancreatic carcinoma cells (PANC-1). J Pineal Res 49:248–255. https://doi.org/10.1111/j.1600-079X.2010.00789.x
Leung M, Tranmer J, Hung E, Korsiak J, Day AG, Aronson KJ (2016) Shift work, chronotype, and melatonin patterns among female hospital employees on day and night shifts. Cancer Epidemiol Biomark Prev 25:830–838. https://doi.org/10.1158/1055-9965.EPI-15-1178
Levoye A, Dam J, Ayoub MA, Guillaume JL, Couturier C, Delagrange P, Jockers R (2006) The orphan GPR50 receptor specifically inhibits MT1 melatonin receptor function through heterodimerization. EMBO J 25:3012–3023
Li Y, Li S, Zhou Y, Meng X, Zhang JJ, Xu DP, Li H (2017) Melatonin for the prevention and treatment of cancer. Oncotarget 8:39896–39921. https://doi.org/10.18632/oncotarget.16379
Lin ZY, Chuang WL (2012) High therapeutic concentration of prazosin up-regulates angiogenic IL6 and CCL2 genes in hepatocellular carcinoma cells. Biomed Pharmacother 66:583–586. https://doi.org/10.1016/j.biopha.2011.09.006
Lin YW, Lee LM, Lee WJ et al (2016) Melatonin inhibits MMP-9 Transactivation and renal cell carcinoma metastasis by suppressing Akt-MAPKs Pathway and NF-kappaB DNA-binding activity. J Pineal Res 60:277–290
Lissoni P (2007) Biochemotherapy with standard chemotherapies plus the pineal hormone melatonin in the treatment of advanced solid neoplasms. Pathol Biol 55:201–204
Lissoni P, Chilelli M, Villa S, Cerizza L, Tancini G (2003) Five years survival in metastatic non-small cell lung cancer patients treated with chemotherapy alone or chemotherapy and melatonin: a randomized trial. J Pineal Res 35:12–15
Liu L, Ying XuY, Reiter RJ (2013a) Melatonin inhibits the proliferation of human osteosarcoma cell line MG-63. Bone 55(2):432–438. https://doi.org/10.1016/j.bone.2013.02.021
Liu R, Fu A, Hoffman AE, Zheng T, Zhu Y (2013b) Melatonin enhances DNA repair capacity possibly by affecting genes involved in DNA damage responsive pathways. BMC Cell Biol 14:1. https://doi.org/10.1186/1471-2121-14-1
Liu J, Clough SJ, Hutchinson AJ, Adamah-Biassi EB, Popovska-Gorevski M, Dubocovich ML (2016a) MT1 and MT2 melatonin receptors: a therapeutic perspective. Annu Rev Pharmacol Toxicol 56:361–383. https://doi.org/10.1146/annurev-pharmtox-010814-124742
Liu L, Xu Y, Reiter RJ, Pan Y, Chen D, Liu Y et al (2016b) Inhibition of ERK1/2 signaling pathway is involved in melatonin’s antiproliferative effect on human MG-63 osteosarcoma cells. Cell Physiol Biochem 39:2297–2307. https://doi.org/10.1159/000447922
Long F, Dong C, Jiang K, Xu Y, Chi X, Sun D, Liang R, Gao Z, Shao S, Wang L (2017) Melatonin enhances the anti-tumor effect of sorafenib via AKT/p27-mediated cell cycle arrest in hepatocarcinoma cell lines. RSC Adv 7:21342–21351. https://doi.org/10.1039/c7ra02113e
Lu Y, Yi Y, Liu P, Wen W, James M, Wang D, You M (2007) Common human cancer genes discovered by integrated gene-expression analysis. PLoS One 2:e1149
Lu J-J, Fu L, Tang Z, Zhang C, Qin L et al (2016) Melatonin inhibits AP-2β/hTERT, NF-κB/COX-2 and Akt/ERK and activates caspase/Cyto C signaling to enhance the antitumor activity of berberine in lung cancer cells. Oncotarget 7(3):2985–3001. https://doi.org/10.18632/oncotarget.6407
Lupowitz Z, Zisapel N (1999) Hormonal interactions in human prostate tumor LNCaP cells. J Steroid Biochem Mol Biol 68(1–2):83–88. https://doi.org/10.1016/s0960-0760(98)00164-2
Majidinia M, Sadeghpour A, Mehrzadi S, Reiter RJ, Khatami N, Yousefi B (2017) Melatonin: a pleiotropic molecule that modulates DNA damage response and repair pathways. J Pineal Res 63:e12416
Malhotra S, Sawhney G, Pandhi P (2004) The therapeutic potential of melatonin: a review of the science. Med Gen Med 6(2):46
Malmlöf M, Roudier E, Högberg J, Stenius U (2007) MEK-ERK-mediated phosphorylation of Mdm2 at Ser-166 in hepatocytes. Mdm2 is activated in response to inhibited Akt signaling. J Biol Chem 282:2288–2296
Mao L, Yuan L, Slakey LM et al (2010) Inhibition of breast cancer cell invasion by melatonin is mediated through regulation of the p38 mitogen-activated protein kinase signaling pathway. Breast Cancer Res 12:R107
Mao L, Summers W, Xiang S et al (2016) Melatonin represses metastasis in Her2-postive human breast cancer cells by suppressing RSK2 expression. Mol Cancer Res 14:1159–1169
Marelli MM, Limonta P, Maggi R, Motta M, Moretti RM (2000) Growth-inhibitory activity of melatonin on human androgen-independent DU 145 prostate cancer cells. Prostate 45(3):238–244
Marnett LJ (1987) Peroxyl free radicals: potential mediators of tumor initiation and promotion. Carcinogenesis 8:1365–1373
Martinez-Campa C, Gonzalez A, Mediavilla MD, Alonso-Gonzalez C, Alvarez-Garcia V, Sanchez-Barcelo EJ, Cos S (2009) Melatonin inhibits aromatase promoter expression by regulating cyclooxygenases expression and activity in breast cancer cells. Br J Cancer 101:1613–1619
Martín-Renedo J, Mauriz JL, Jorquera F, Ruiz-Andrés O, González P, González-Gallego J (2008) Melatonin induces cell cycle arrest and apoptosis in hepatocarcinoma HepG2 cell line. J Pineal Res 45:532–540. https://doi.org/10.1111/j.1600-079X.2008.00641.x
Masoud GN, Li W (2015) HIF-1alpha pathway: role, regulation and intervention for cancer therapy. Acta Pharm Sin B 5:378–389
Masson N, Ratcliffe PJ (2014) Hypoxia signaling pathways in cancer metabolism: the importance of co- selecting interconnected physiological pathways. Cancer Metab 2(3):1–17
Mattam U, Jagota A (2014) Differential role of melatonin in restoration of age-induced alterations in daily rhythms of expression of various clock genes in suprachiasmatic nucleus of male Wistar rats. Biogerontol 15:257–268
Matuszak Z, Bilska MA, Reszkat KJ, Chignell CF, Bilski P (2003) Interaction of singlet molecular oxygen with melatonin and related indoles. Photochem Photobiol 78:449–455
Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ (1999) The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399:271–275
Maya-Mendoza A, Ostrakova J, Kosar M, Hall A, Duskova P, Mistrik M, Merchut-Maya JM, Hodny Z, Bartkova J, Christensen C et al (2015) Myc and Ras oncogenes engage different energy metabolism programs and evoke distinct patterns of oxidative and DNA replication stress. Mol Oncol 9(3):601–616
Mediavilla MD, Cos S, Sanchez-Barcelo EJ (1999) Melatonin increases p53 and p21WAF1 expression in MCF-7 human breast cancer cells in vitro. Life Sci 65:415–420
Menéndez-Menéndez J, Martínez-Campa C (2018) Melatonin: an anti-tumor agent in hormone-dependent cancers. Int J Endocrinol Article ID 3271948. https://doi.org/10.1155/2018/3271948
Min C, Eddy SF, Sherr DH et al (2008) NF-kappaB and epithelial to mesenchymal transition of cancer. J Cell Biochem 104:733–744
Molis TM, Spriggs LL, Jupiter Y, Hill SM (1995) Melatonin modulation of estrogen-regulated proteins, growth factors, and proto-oncogenes in human breast cancer. J Pineal Res 18(2):93–103
Morales-Santana S et al (2019) An overview of the polymorphisms of circadian genes associated with endocrine cancer. Front Endocrinol 10:104. https://doi.org/10.3389/fendo.2019.00104
Moretti RM, Marelli MM, Maggi R, Dondi D, Motta M, Limonta P (2000) Antiproliferative action of melatonin on human prostate cancer LNCaP cells. Oncol Rep 7(2):347–351
Morgan PJ, Barrett P, Howell HE, Helliwell R (1994) Melatonin receptor localisation, molecular pharmacology and physiological significance. Neurochem Int 24:101–146
Mukherjee A, Haldar C (2014) Photoperiodic regulation of melatonin membrane receptor (MT1R) expression and steroidogenesis in testis of adult golden hamster, Mesocricetus auratus. J Photochem Photobiol B Biol 140:374–380
Murata M, Thanan R, Ma N, Kawanishi S (2012) Role of nitrative and oxidative DNA damage in inflammation-related carcinogenesis. J Biomed Biotechnol. https://doi.org/10.1155/2012/623019
Nagorny C, Lyssenko V (2012) Tired of diabetes genetic? Circadian rhythms and diabetes: the MTNR1B story? Curr Diab Rep 12(6):667–672
Najafi M, Salehi E, Farhood B, Nashtaei MS et al (2019) Adjuvant chemotherapy with melatonin for targeting human cancers: a review. J Cell Physiol 234:2356–2372. https://doi.org/10.1002/jcp.27259
Norsa A, Martino V (2006) Somatostatin, retinoids, melatonin, vitamin D, bromocriptine, and cyclophosphamide in advanced non-small-cell lung cancer patients with low performance status. Cancer Biother Radiopharm 21:68–73
Nosjean O, Ferro M, Coge F, Beauverger P, Henlin JM, Lefoulon F et al (2000) Identification of the melatonin-binding site MT3 as the quinone reductase 2. J Biol Chem 275(40):31311–31317
Nowfar S, Teplitzky SR, Melancon K et al (2002) Tumor prevention by 9-cisretinoic acid in the N-nitroso-N-methylurea model of mammary carcinogenesis is potentiated by the pineal hormone melatonin. Breast Cancer Res Treat 72:33–43
Onseng K, Johns NP, Khuayjarernpanishk T, Subongkot S, Priprem A, Hurst C, Johns J (2017) Beneficial effects of adjuvant melatonin in minimizing oral mucositis complications in head and neck cancer patients receiving concurrent chemoradiation. J Altern Complement Med 23:957–963
Ortiz-Lopez L, Morales-Mulia S, Ramirez-Rodriguez G et al (2009) ROCK-regulated cytoskeletal dynamics participate in the inhibitory effect of melatonin on cancer cell migration. J Pineal Res 46:15–21
Osanai K, Kobayashi Y, Otsu M, Izawa T, Sakai K, Iwashita M (2017) Ramelteon, a selective MT1/T2 receptor agonist, suppresses the proliferation and invasiveness of endometrial cancer cells. Hum Cell 30:209–215. https://doi.org/10.1007/s13577-017-0169-7
Ostrin LA (2019) Ocular and systemic melatonin and the influence of light exposure. Clin Exp Optom 102:99–108
Pandi-Perumal SR, Trakht I, Srinivasan V et al (2008) Physiological effects of melatonin: role of melatonin receptors and signal transduction pathways. Prog Neurobiol 85:335–353
Papantoniou K, Pozo OJ, Espinosa A, Marcos J, Castaño-Vinyals G, Basagaña X, Juanola Pagès E, Mirabent J, Martín J, Such Faro P et al (2015) Increased and mistimed sex hormone production in night shift workers. Cancer Epidemiol Biomark Prev 24:854–863. https://doi.org/10.1158/1055-9965.EPI-14-1271
Parameyong A, Charngkaew K, Govitrapong P, Chetsawang B (2013) Melatonin attenuates methamphetamine-induced disturbances in mitochondrial dynamics and degeneration in neuroblastoma SH-SY5Y cells. J Pineal Res 55:313–323
Pariente R, Bejarano I, Espino J, Rodríguez AB, Pariente JA (2017) Participation of MT3 melatonin receptors in the synergistic effect of melatonin on cytotoxic and apoptotic actions evoked by chemotherapeutics. Cancer Chemother Pharmacol 80:985–998. https://doi.org/10.1007/s00280-017-3441-3
Pariente R, Bejarano I, Rodríguez AB, Pariente JA, Espino J (2018) Melatonin increases the effect of 5-fluorouracil-based chemotherapy in human colorectal adenocarcinoma cells in vitro. Mol Cell Biochem 440:43–51. https://doi.org/10.1007/s11010-017-3154-2
Park J-W, Suh S-II, Hwang M-S, Baek W (2009) Melatonin down-regulates HIF-1α expression through inhibition of protein translation in prostate cancer cells. J Pineal Res 46(4):415–421. https://doi.org/10.1111/j.1600-079X.2009.00678.x
Park SY, Jang WJ, Yi EY, Jang JY, Jung Y, Jeong JW, Kim YJ (2010) Melatonin suppresses tumor angiogenesis by inhibiting HIF-1alpha stabilization under hypoxia. J Pineal Res 48(2):178–184. https://doi.org/10.1111/j.1600-079x.2009.00742.x
Park MT, Kim MJ, Suh Y, Kim RK, Kim H, Lim EJ, Yoo KC, Lee GH, Kim Yh, Hwang SG et al (2014) Novel signaling axis for ROS generation during KRas-induced cellular transformation. Cell Death Differ 21(8):1185–1197
Parmar P, Limson J, Nyokong T, Daya S (2002) Melatonin protects against copper-mediated free radical damage. J Pineal Res 32:237–242
Pauley SM (2004) Lighting for the human circadian clock: recent research indicates that lighting has become a public health issue. Med Hypotheses 63:588–596. https://doi.org/10.1016/j.mehy.2004.03.020
Peters MG, Farias E, Colombo L et al (2003) Inhibition of invasion and metastasis by glypican-3 in a syngeneic breast cancer model. Breast Cancer Res Treat 80:221–232
Petranka J, Baldwin W, Biermann J, Jayadev S, Barrett JC, Murphy E (1999) The oncostatic action of melatonin in an ovarian carcinoma cell line. J Pineal Res 26(3):129–136. https://doi.org/10.1111/j.1600-079X.1999.tb00574.x
Pett JP et al (2016) Feedback loops of the mammalian circadian clock constitute repressilator. PLoS Comput Biol 12:e1005266
Poole EM, Schernhammer ES, Tworoger SS (2011) Rotating night shift work and risk of ovarian cancer. Cancer Epidemiol Biomark Prev 20(5):934–938
Potter GDM (2016) Circadian rhythm and sleep disruption: causes, metabolic consequences, and countermeasures. Endocr Rev 37(6):584–608
Pourhanifeh MH, Sharifi M, Reiter RJ, Davoodabadi A, Asemi Z (2019) Melatonin and non-small cell lung cancer: new insights into signaling pathways. Cancer Cell Int 19:131. https://doi.org/10.1186/s12935-019-0853-7
Proietti S, Cucina A, D’Anselmi F, Dinicola S, Pasqualato A, Lisi E, Bizzarri M (2011) Melatonin and vitamin D3 synergistically down-regulate Akt and MDM2 leading to TGFβ-1-dependent growth inhibition of breast cancer cells. J Pineal Res 50:150–158
Proietti S, Cucina A, Reiter RJ, Bizzarri M (2013) Molecular mechanisms of melatonin’s inhibitory actions on breast cancers. Cell Mol Life Sci 70:2139–2157. https://doi.org/10.1007/s00018-012-1161-8
Proietti S, Cucina A, Dobrowolny G, D’Anselmi F, Dinicola S, Masiello MG et al (2014) Melatonin down-regulates MDM2 gene expression and enhances p53 acetylation in MCF-7 cells. J Pineal Res 57:120–129. https://doi.org/10.1111/jpi.12150
Qin F, Zhang J, Zan L et al (2015) Inhibitory effect of melatonin on testosterone synthesis is mediated via GATA-4/SF-1 transcription factors. Reproduct Biomed Online 31(5):638–646
Radi R, Peluffo G, Alvarez MN, Naviliat M, Cayota A (2001) Unraveling peroxynitrite formation in biological systems. Free Radic Biol Med 30:463–488
Rato AG, Pedrero JG, Martínez MA, Del Rio B, Lazo PS, Ramos S (1999) Melatonin blocks the activation of estrogen receptor for DNA binding. FASEB J 13(8):857–868
Reiter RJ, Acuna-Castroviejo D, Tan DX, Burkhardt S (2001) Free radical-mediated molecular damage: mechanisms for the protective actions of melatonin in the central nervous system. Ann N Y Acad Sci 939:200–215
Reiter RJ, Rosales-Corral SA, Tan DX, Acuna-Castroviejo D, Qin L, Yang SF et al (2017) Melatonin, a full service anti-cancer agent: inhibition ofinitiation, progression and metastasis. Int J Mol Sci 18:e843. https://doi.org/10.3390/ijms18040843
Rimler A, Jockers R, Lupowitz Z, Sampson SR, Zisapel N (2005) Differential effects of melatonin and its downstream effector PKCalpha on subcellular localization of RGS proteins. J Pineal Res 40:144–152. https://doi.org/10.1111/j.1600-079X.2005.00290.x
Rimler A, Jockers R, Lupowitz Z, Zisapel N (2007) Gi and RGS proteins provide biochemical control of androgen receptor nuclear exclusion. J Mol Neurosci 31:1–12
Romero et al (2014) A review of metal-catalyzed molecular damage: protection by melatonin. J Pineal Res 56(4):343–370. https://doi.org/10.1111/jpi.12132
Rubio S, Estévez F, Cabrera J et al (2007) Inhibition of proliferation and induction of apoptosis by melatonin in human myeloid HL-60 cells. J Pineal Res 42:131–138
Sakatani A, Sonohara F, Goel A (2019) Melatonin-mediated downregulation of thymidylate synthase as a novel mechanism for overcoming 5-fluorouracil associated chemoresistance in colorectal cancer cells. Carcinogenesis 40(3):422–431. https://doi.org/10.1093/carcin/bgy186
Samanta S (2020) Physiological and pharmacological perspectives of melatonin. https://doi.org/10.1080/13813455.2020.1770799
Samanta S, Dassarma B, Jana S, Rakshit S, Saha SA (2018) Hypoxia inducible factor-1 (HIF-1) and cancer progression: a comprehensive review. Indian J Cancer Edu Res 6(1):94–109
Sánchez-Hidalgo M, Lee M, de la Lastra CA et al (2012) Melatonin inhibits cell proliferation and induces caspase activation and apoptosis in human malignant lymphoid cell lines. J Pineal Res 53:366–373
Sandyk R, Anastasiadis PG, Anninos PA, Tsagas N (1991) Is the pineal gland involved in the pathogenesis of endometrial carcinoma. Int J Neurosci 62(1–2):89–96
Santoro R, Marani M, Blandino G, Muti P, Strano S (2012) Melatonin triggers p53 Ser phosphorylation and prevents DNA damage accumulation. Oncogene 31:2931–2942
Schernhammer ES, Schulmeister K (2007) Melatonin and cancer risk: does light at night compromise physiologic cancer protection by lowering serum melatonin levels? Br J Cancer 90:941–943. https://doi.org/10.1038/sj.bjc.6601626
Schernhammer ES, Laden F, Speizer FE et al (2001) Rotating night shifts and risk of breast cancer in women participating in the nurses’ health study. J Nat Cancer Inst 93(20):1563–1568
Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I et al (2003) Night-shift work and risk of colorectal cancer in the nurses’ health study. J Natl Cancer Inst 95(11):825–828
Schernhammer ES, Giobbie-Hurder A, Gantman K, Savoie J, Scheib R, Parker LM, Chen WY (2012) A randomized controlled trial of oral melatonin supplementation and breast cancer biomarkers. Cancer Causes Control 23:609–616
Schock H, Surcel HM, Zeleniuch-Jacquotte A et al (2014) Early pregnancy sex steroids and maternal risk of epithelial ovarian cancer. Endocr Relat Cancer 21(6):831–844
Seely D, Wu P, Fritz H, Kennedy DA, Tsui T, Seely AJ, Mills E (2012) Melatonin as adjuvant cancer care with and without chemotherapy: a systematic review and meta-analysis of randomized trials. Integr Cancer Ther 11:293–303
Semenza GL (2010) Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene 29:625–634
Shen CJ, Chang CC, Chen YT, Lai CS, Hsu YC (2016) Melatonin suppresses the growth of ovarian cancer cell lines (OVCAR-429 and PA-1) and potentiates the effect of g1 arrest by targeting CDKs. Int J Mol Sci 17:e176. https://doi.org/10.3390/ijms17020176
Shiu SYW, Xi SC, Xu JN, Mei L, Pang SF, Yao KM, Wong JTY (2000) Inhibition of malignant trophoblastic cell proliferation in vitro and in vivo by melatonin. Life Sci 67(17):2059–2074. https://doi.org/10.1016/S0024-3205(00)00792-X
Shiu SYW, Leung WY, Tam CW, Liu VWS, Yao K-M (2013) Melatonin MT1 receptor-induced transcriptional up-regulation of p27Kip1 in prostate cancer antiproliferation is mediated via inhibition of constitutively active nuclear factor kappa B (NF-κB): potential implications on prostate cancer chemoprevention and therapy. J Pineal Res 54(1):69–79
Siepka SM et al (2007) Circadian mutant overtime reveals F-box protein FBXL3 regulation of cryptochrome and period gene expression. Cell 129:1011–1023. https://doi.org/10.1016/j.cell.2007.04.030
Sies H (1997) Oxidative stress: oxidants and antioxidants. Exp Physiol 82:291–295. https://doi.org/10.1113/expphysiol.1997.sp004024
Siu AW, Ortiz GG, Benitez-King G, To CH, Reiter RJ (2004) Effect of melatonin on the nitric oxide treated retina. Br J Ophthalmol 88:1078–1081
Sofic E, Rimpapa Z, Kundurovic Z et al (2005) Antioxidant capacity of the neurohormone melatonin. J Neural Transm 112:349–358
Song G, Yoon KA, Chi H, Roh J, Kim JH (2016) Decreased concentration of serum melatonin in nighttime compared with daytime female medical technologists in South Korea. Chronobiol Int 33:1305–1310. https://doi.org/10.1080/07420528.2016.1199562
Song J, Ma S-J, Luo J-H, Zhang H, Wang R-X, Liu H, Li L, Zhang Z-G, Zhou R-X (2018) Melatonin induces the apoptosis and inhibits the proliferation of human gastric cancer cells via blockade of the AKT/MDM2 pathway. Oncol Rep 39:1975–1983. https://doi.org/10.3892/or.2018.6282
Srinivas US, Tan BWQ, Vellayappan BA, Jeyasekharan AD (2019) ROS and the DNA damage response in cancer. Redox Biol 25:101084. https://doi.org/10.1016/j.redox.2018.101084
Stevens RG (1987) Electric power use and breast cancer: a hypothesis. Am J Epidemiol 125(4):556–561
Straif K, Baan R, Grosse Y, Secretan B, El Ghissassi F, Bouvard V et al (2007) Carcinogenicity of shift-work, painting, and fire-fighting. Lancet Oncol 8:1065–1066. https://doi.org/10.1016/S1470-2045(07)70373-X
Su S-C, Hsieh M-J, Yang W-E, Chung W-H, Reiter RJ, Yang S-F (2017) Cancer metastasis: mechanisms of inhibition by melatonin. J Pineal Res 62:e12370. https://doi.org/10.1111/jpi.12370
Suofu Y, Li W, Jean-Alphonse FG, Jia J, Khattar NK, Li J, Baranov SV, Leronni D, Mihalik AC, He Y et al (2017) Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. Proc Natl Acad Sci USA 114:E7997–E8006. https://doi.org/10.1073/pnas.1705768114
Tam CW, Mo CW, Yao K, Shiu SY (2007) Signaling mechanisms of melatonin in antiproliferation of hormone-refractory 22Rv1 human prostate cancer cells: implications for prostate cancer chemoprevention. J Pineal Res 42:191–202
Tamarkin L, Danforth D, Lichter A et al (1982) Decreased nocturnal plasma melatonin peak in patients with estrogen receptor positive breast cancer. Science 216(4549):1003–1005
Tamura H, Nakamura Y, Korkmaz A et al (2009) Melatonin and the ovary: physiological and pathophysiological implications. Fertil Steril 92(1):328–343
Tan D-X, Reiter RJ, Manchester LC et al (2002) Chemical and physical properties and potential mechanisms: melatonin as a broad spectrum antioxidant and free radical scavenger. Curr Top Med Chem 2:181–197
Tan D-X, Manchester LC, Esteban-Zubero E, Zhou Z, Reiter RJ (2015) Melatonin as a potent and inducible endogenous antioxidant: synthesis and metabolism. Molecules 20:18886–18906. https://doi.org/10.3390/molecules201018886
Tan D-X, Manchester LC, Qin L, Reiter RJ (2016) Melatonin: a mitochondrial targeting molecule involving mitochondrial protection and dynamics. Int J Mol Sci 17:2124
Tan D-X, Xu B, Zhou X, Reiter RJ (2018) Pineal calcification, melatonin production, aging, associated health consequences and rejuvenation of the pineal gland. Molecules 23:301. https://doi.org/10.3390/molecules23020301
Thomson PA, Wray NR, Thomson AM, Dunbar DR, Grassie MA, Condie A, Walker MT, Smith DJ, Pulford DJ, Mur W, Blackwood DH, Porteus DJ (2005) Sex-specific association between bipolar affective disorder in women and GPR50, an X-linked orphan G protein-coupled receptor. Mol Psychiatry 10:470–478
Todisco M (2006) Relapse of high-grade non-Hodgkin’s lymphoma after autologous stem cell transplantation: a case successfully treated with cyclophosphamide plus somatostatin, bromocriptine, melatonin, retinoids, and ACTH. Am J Ther 13:556–557
Todisco M (2007) Low-grade non-Hodgkin lymphoma at advanced stage: a case successfully treated with cyclophosphamide plus somatostatin, bromocriptine, retinoids, and melatonin. Am J Ther 14:113–115
Todisco M, Casaccia P, Rossi N (2001) Cyclophosphamide plus somatostatin bromocriptin, retinoids, melatonin and ACTH in the treatment of low-grade non-Hodgkin’s lymphomas at advanced stage: results of a phase II trial. Cancer Biother Radiopharm 16:171–177
Tomas-Zapico C, Coto-Montes A (2005) A proposed mechanism to explain the stimulatory effect of melatonin on antioxidative enzymes. J Pineal Res 39:99–104
Trubiani O, Recchioni R, Moroni F et al (2005) Melatonin provokes cell death in human B-lymphoma cells by mitochondrial-dependent apoptotic pathway activation. J Pineal Res 39:425–431
Valizadeh M, Shirazi A, Izadi P, Tavakkoly Bazzaz J, Rezaeejam H (2017) Expression levels of two dna repair-related genes under 8 gy ionizing radiation and 100 mg/kg melatonin delivery in rat peripheral blood. J Biomed Phys Eng 7:27–36
Vijayalaxmi TX, Thomas CR Jr, Reiter RJ, Herman TS (2002) Melatonin: from basic research to cancer treatment clinics. J Clin Oncol 20(10):2575–2601
von Gall C, Weaver DR, Moek J, Jilg A et al (2005) Melatonin plays a crucial role in the regulation of rhythmic clock gene expression in the mouse pars tuberalis. Ann NY Acad Sci 1040:508–511
Vriend J, Reiter RJ (2015) Melatonin feedback on clock genes: a theory involving the proteasome. J Pineal Res 58:1–11. https://doi.org/10.1111/jpi.12189
Vriend J, Reiter RJ (2016) Melatonin and the von Hippel–Lindau/HIF-1 oxygen sensing mechanism: a review. Biochem Biophys Acta 1865(2):176–183. https://doi.org/10.1016/j.bbcan.2016.02.004
Wang J, Xiao X, Zhang Y et al (2012) Simultaneous modulation of COX-2, p300, Akt, and Apaf-1 signaling by melatonin to inhibit proliferation and induce apoptosis in breast cancer cells. J Pineal Res 53:77–90
Wang R-X, Liu H, Xu L, Zhang H, Zhou R-X (2015) Involvement of nuclear receptor RZR/RORγ in melatonin-induced HIF-1α inactivation in SGC-7901 human gastric cancer cells. Oncol Rep 34:2541–2546. https://doi.org/10.3892/or.2015.4238
Wang RX, Liu H, Xu L, Zhang H, Zhou RX (2016) Melatonin downregulates nuclear receptor RZR/ROR expression causing growth-inhibitory and anti-angiogenesis activity in human gastric cancer cells in vitro and in vivo. Oncol Lett 12:897–903
Wei JY, Li WM, Zhou LL, Lu QN, He W (2015) Melatonin induces apoptosis of colorectal cancer cells through HDAC4 nuclear import mediated by CaMKII inactivation. J Pineal Res 58:429–438. https://doi.org/10.1111/jpi.12226
Wiesenberg I, Missbach M, Carlberg C (1998) The potential role of the transcription factor RZR/ROR as a mediator of nuclear melatonin signaling. Restor Neurol Neurosci 12:143–150
Wilson ST, Blask DE, Lemus-Wilson AM (1992) Melatonin augments the sensitivity of MCF-7 human breast cancer cells to tamoxifen in vitro. J Clin Endocrinol Metab 75:669–670
Xu CS, Wang ZF, Huang XD et al (2015) Involvement of ROS-alpha vs beta 3 integrin-FAK/Pyk2 in the inhibitory effect of melatonin on U251 glioma cell migration and invasion under hypoxia. J Transl Med 13:95
Yan JJ, Shen F, Wang K, Wu MC (2002) Patients with advanced primary hepatocellular carcinoma treated by melatonin and transcatheter arterial chemoembolization: a prospective study. Hepatobiliary Pancreat Dis Int 1:183–186
Yang XO, Chang SH, Park H, Nurieva R, Shah B, Acero L, Wang YH, Schluns KS, Broaddus RR, Zhu Z, Dong C (2008a) Regulation of inflammatory responses by IL-17F. J Exp Med 205:1063–1075
Yang JY, Zong CS, Xia W et al (2008b) ERK promotes tumorigenesis by inhibiting FOXO3a via MDM2-mediated degradation. Nat Cell Biol 10:138–148
Yi C, Zhang Y, Yu Z, Xiao Y, Wang J et al (2014) Melatonin enhances the anti-tumor effect of fisetin by inhibiting COX-2/iNOS and NF-κB/p300 signaling pathways. PLoS One 9(7):e99943
Zatta P, Tognon G, Carampin P (2003) Melatonin prevents free radical formation due to the interaction between β-amyloid Peptides and metal ions [Al(III), Zn(II), Cu(II), Mn(II), Fe(II)]. J Pineal Res 35:98–103
Zha L, Fan L, Sun G et al (2012) Melatonin sensitizes human hepatoma cells to endoplasmic reticulum stress-induced apoptosis. J Pineal Res 52:322–331
Zhang Q, Yang H (2012) The roles of VHL-dependent ubiquitination in signaling and cancer. Front Oncol 2:35
Zhao M, Wan J, Zeng K et al (2016) The reduction in circulating melatonin level may contribute to the pathogenesis of ovarian cancer: a retrospective study. J Cancer 7(7):831–836
Zhao D, Yu Y, Shen Y, Liu Q, Zhao Z, Sharma R, Reiter RJ (2019) Melatonin synthesis and function: evolutionary history in animals and plants. Front Endocrinol 10:249. https://doi.org/10.3389/fendo.2019.00249
Zhou Q, Gui S, Zhou Q et al (2014) Melatonin inhibits the migration of human lung adenocarcinoma A549 cell lines involving JNK/MAPK pathway. PLoS One 9:e101132
Zhu Y, McAvoy S, Kuhn R, Smith DI (2006) RORA, a large common fragile site gene, is involved in cellular stress response. Oncogene 25:2901–2908
Acknowledgements
The author is grateful to Midnapore College, Midnapore, West Bengal, India, for providing all kinds of facilities to prepare this manuscript.
Funding
No financial grant was available. This review article was self supported by the author.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author has no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Samanta, S. Melatonin: an endogenous miraculous indolamine, fights against cancer progression. J Cancer Res Clin Oncol 146, 1893–1922 (2020). https://doi.org/10.1007/s00432-020-03292-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-020-03292-w