Abstract
Purpose
The present study aims to determine whether co-targeting PI3K/Akt and MAPK/ERK pathways in human hypopharyngeal squamous cell carcinoma (HSCC) is a potential anticancer strategy.
Methods
We retrospectively analyzed the clinical data of HSCC patients, and the phosphorylation status of Akt and Erk in HSCC and tumor adjacent tissues was evaluated by immunohistochemistry. MTT and colony formation assay were performed to determine the anti-proliferative effect of PI3K/mTOR inhibitor GDC-0980 and MEK inhibitor Refametinib on HSCC cell line Fadu. Wound-healing and Transwell migration assay were used to analyze the anti-migrative capability of the two drugs. The involved anti-tumor mechanism was explored by flow cytometry, qRT-PCR and western blot. The combinational anticancer effect of GDC-0980 and Refametinib was evaluated according to Chou and Talalay’s method.
Results
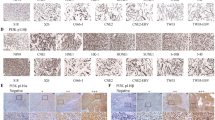
The levels of p-Akt and p-Erk were increased significantly with the progression of clinical stage of HSCC, suggesting PI3K/Akt and MAPK/ERK pathways might be associated with HSCC occurrence and progression. Furthermore, both GDC-0980 and Refametinib showed obvious antitumor effects on FaDu cells. Treatment by the two drugs arrested FaDu cell cycle progression in G1 phase, with reduction of cyclin D1 and p-Rb, in contrast to enhancement of p27. GDC-0980 inhibited FaDu cell migration and reduced metastasis related proteins including p-PKCζ, p-Integrin β1 and uPA. Combination use of GDC-0980 and Refametinib exhibited strong synergistic anti-tumor effect.
Conclusion
Dual inhibition of PI3K/Akt and MAPK/ERK pathway by GDC-0980 and Refametinib might be a promising treatment strategy for HSCC patients.







Similar content being viewed by others
References
Aksamitiene E, Kiyatkin A, Kholodenko BN (2012) Cross-talk between mitogenic Ras/MAPK and survival PI3K/Akt pathways: a fine balance. Biochem Soc Trans 40:139–146
Bancroft CC, Chen Z, Dong G et al (2001) Coexpression of proangiogenic factors IL-8 and VEGF by human head and neck squamous cell carcinoma involves coactivation by MEK-MAPK and IKK-NF-kappaB signal pathways. Clin Cancer Res 7:435–442
Cargnello M, Roux PP (2011) Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev 75:50–83
Chaffer CL, Weinberg RA (2011) A perspective on cancer cell metastasis. Science 331:1559–1564
Dan S, Okamura M, Seki M (2010) Correlating phosphatidylinositol 3-kinase inhibitor efficacy with signaling pathway status: in silico and biological evaluations. Cancer Res 70:4982–4994
Davies L, Welch HG (2006) Epidemiology of head and neck cancer in the United States. Otolaryngol Head Neck Surg 135:451–457
De Luca A, Maiello MR, D’Alessio A et al (2012) The RAS/RAF/MEK/ERK and the PI3K/AKT signalling pathways: role in cancer pathogenesis and implications for therapeutic approaches. Expert Opin Ther Targets 16:S17–S27
Dutta S, Bandyopadhyay C, Bottero V et al (2014) Angiogenin interacts with the plasminogen activation system at the cell surface of breast cancer cells to regulate plasmin formation and cell migration. Mol Oncology 8:483–507
Eleveld TF, Oldridge DA, Bernard V et al (2015) Relapsed neuroblastomas show frequent RAS-MAPK pathway mutations. Nat Genet 47:864–871
Fedorov SN, Shubina LK, Bode AM et al (2007) Dactylone inhibits epidermal growth factor-induced transformation and phenotype expression of human cancer cells and induces G1-S arrest and apoptosis. Cancer Res 67:5914–5920
Hirai T, Chida K (2003) Protein kinase Czeta (PKCzeta): activation mechanisms and cellular functions. J Biochem 133:1–7
Isoyama S, Yoshimi H, Dan S et al (2012) Development of an immunohistochemical protein quantification system in conjunction with tissue microarray technology for identifying predictive biomarkers for phosphatidylinositol 3-kinase inhibitors. Biol Pharm Bull 35:1607–1613
Kundu SK, Nestor M (2012) Targeted therapy in head and neck cancer. Tumour Biol 33:707–721
Kuo P, Sosa JA, Burtness BA et al (2016) Treatment trends and survival effects of chemotherapy for hypopharyngeal cancer: analysis of the national cancer data base. Cancer 122:1853–1860
Kwon DI, Miles BA (2019) Education Committee of the American Head and Neck Society (AHNS). Hypopharyngeal carcinoma: do you know your guidelines? Head Neck 41:569–576
Liang J, Slingerland JM (2003) Multiple roles of the PI3K/PKB (Akt) pathway in cell cycle progression. Cell Cycle 2:339–345
Liu Y, Wang B, Wang J et al (2009) Down-regulation of PKCzeta expression inhibits chemotaxis signal transduction in human lung cancer cells. Lung Cancer 63:210–218
Machiels JP, Schmitz S (2011) New advances in targeted therapies for squamous cell carcinoma of the head and neck. Anticancer Drugs 22:626–633
Malumbres M, Barbacid M (2009) Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer 9:153–166
Martini M, Ciraolo E, Gulluni F et al (2013) Targeting PI3K in cancer: any good news? Front Oncol 3:108
Massacesi C, Di Tomaso E, Urban P et al (2016) PI3K inhibitors as new cancer therapeutics: implications for clinical trial design. Onco Targets Ther 9:203–210
Mayer IA, Arteaga CL (2016) The PI3K/AKT pathway as a target for cancer treatment. Ann Rev Med 67:11–28
McCubrey JA, Steelman LS, Abrams SL et al (2008) Targeting survival cascades induced by activation of Raf/Raf/MEK/ERK, PI3K/PTEN/Akt/mTOR and Jak/STAT pathways for effective leukemia therapy. Leukemia 22:708–722
Mendoza MC, Er EE, Blenis J (2011) The Ras-ERK and PI3K-mTOR pathways: crosstalk and compensation. Trends Biochem Sci 36:320–328
Newman JR, Connolly TM, Illing EA et al (2015) Survival trends in Hypopharyngeal cancer: a population based review. Laryngoscope 125:6249
Parfenov M, Pedamallu CS, Gehlenborg N et al (2014) Characterization of HPV and host genome interactions in primary head and neck cancers. Proc Natl Acad Sci USA 111:15544–15549
Peng X, Liu Y, Peng X et al (2018) Clinical features and the molecular biomarkers of olfactory neuroblastoma. Pathol Res Pract 214:1123–1129
Powles T, Lackner MR, Oudard S et al (2016) Randomized open-label phase II trial of apitolisib (GDC-0980), a novel inhibitor of the PI3K/mammalian target of rapamycin pathway, versus everolimus in patients with metastatic renal cell carcinoma. J Clin Oncol 34:1660–1668
Ren H, Guo H, Thakur A et al (2016) Blockade efficacy of MEK/ERK-dependent autophagy enhances PI3K/Akt inhibitor NVP-BKM120′s therapeutic effectiveness in lung cancer cells. Oncotarget 7:67277–67287
Renshaw J, Taylor KR, Bishop R et al (2013) Dual blockade of the PI3K/AKT/mTOR (AZD8055) and RAS/MEK/ERK (AZD6244) pathways synergistically inhibits rhabdomyosarcoma cell growth in vitro and in vivo. Clin Cancer Res 19:5940–5951
Shaw RJ, Cantley LC (2006) Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature 441:424–430
Siegel R, Naishadham D, Jemal A (2013) Cancer statistics. CA Cancer J Clin 63:11–30
Steelman LS, Chappell WH, Abrams SL et al (2011) Roles ofthe Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR pathways in controlling growth and sensitivity to therapy-implications for cancer and aging. Aging (Albany NY) 3:192–222
Sun R, Gao P, Chen L et al (2005) Protein kinase C zeta is required for epidermal growth factor-induced chemotaxis of human breast cancer cells. Cancer Res 65:1433–1441
Thorpe LM, Yuzugullu H, Zhao JJ (2015) PI3K in cancer: divergent roles of isoforms, modes of activation and therapeutic targeting. Nat Rev Cancer 15:7–24
Van Dort ME, Galbán S, Wang H et al (2015) Dual inhibition of allosteric mitogen-activated protein kinase (MEK) and phosphatidylinositol 3-kinase (PI3K) oncogenic targets with a bifunctional inhibitor. Bioorg Med Chem 23:1386–1394
Wang H, Wu C, Wan S et al (2013) Shikonin attenuates lung cancer cell adhesion to extracellular matrix and metastasis by inhibiting integrin β1 expression and the ERK1/2 signaling pathway. Toxicology 308:104–112
Wang Y, Qu X, Shen HC et al (2015) Predictive and prognostic biomarkers for patients treated with anti-EGFR agents in lung cancer: a systemic review and meta-analysis. Asian Pac J Cancer Prev 16:4759–4768
Wang R, Zhang Q, Peng X et al (2016a) Stellettin B induces G1 arrest, apoptosis and autophagy in human non-small cell lung cancer A549 cells via blocking PI3K/Akt/mTOR pathway. Sci Rep 6:27071
Wang Y, Liu J, Qiu Y et al (2016b) ZSTK474, a specific class Iphosphatidylinositol 3-kinase inhibitor, induces G1 arrest and autophagy in human breast cancer MCF-7 cells. Oncotarget 7:19897–19909
Wang Z, Wang Y, Zhu S et al (2018) DT-13 inhibits proliferation and metastasis of human prostate cancer cells through blocking PI3K/Akt pathway. Front Pharmacol 9:1450
Williams TM, Flecha AR, Keller P et al (2012) Cotargeting MAPK and PI3K signaling with concurrent radiotherapy as a strategy for the treatment of pancreatic cancer. Mol Cancer Ther 11:1193–1202
Wycliffe ND, Grover RS, Kim PD et al (2007) Hypopharyngeal cancer. Top Magn Reson Imaging 18:243–258
Zhang W, Liu HT (2002) MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res 12:9–18
Zhou Q, Chen Y, Chen X et al (2016) In vitro antileukemia activity of ZSTK474 on K562 and multidrug resistant K562/A02 cells. Int J Biol Sci 12:631–638
Zhou Q, Chen Y, Zhang L et al (2017) Antiproliferative effect of ZSTK474 alone or in combination with chemotherapeutic drugs on HL60 and HL60/ADR cells. Oncotarget 8:39064–39076
Zi D, Zhou ZW, Yang YJ et al (2015) Danusertib induces apoptosis, cell cycle arrest, and autophagy but inhibits epithelial to mesenchymal transition involving PI3K/Akt/mTOR signaling pathway in human ovarian cancer cells. Int J Mol Sci 16:27228–27251
Acknowledgements
The work was supported by grant from National Natural Science Foundation of China (81673464, 81373441), the Grant for Major Project of Tianjin for New Drug Development (17ZXXYSY00050), and the Grant from Natural Science Foundation of Tianjin-Science and Technology (15JCYBJC27500).
Author information
Authors and Affiliations
Contributions
XP, YL and SZ performed the experiments. XP, HL and WJ analyzed the data. ZZ, YQ and MJ prepared the figures. YL, XP and RW wrote the main manuscript. DK revised the manuscript. PL, RW and DK designed the experiments. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiaolin Peng and Yao Liu contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Peng, X., Liu, Y., Zhu, S. et al. Co-targeting PI3K/Akt and MAPK/ERK pathways leads to an enhanced antitumor effect on human hypopharyngeal squamous cell carcinoma. J Cancer Res Clin Oncol 145, 2921–2936 (2019). https://doi.org/10.1007/s00432-019-03047-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-019-03047-2