Abstract
Purpose
The extent to which ≥ 70 year patients with colon cancer benefit from adjuvant chemotherapy in the presence of competing risks remains controversial.
Methods
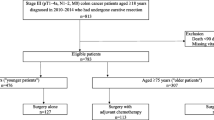
18,937 patients ≥ 70 years with high-risk stage II and stage III colon cancer were retrospectively reviewed from SEER database. Propensity score matching (PSM) was used to adjust for potential baseline confounding. The nomograms were developed based on the competing model to describe the individual probability of colon cancer-specific death (CCSD) and non-CCSD. The subpopulation treatment-effect pattern plot (STEPP) was used to estimate the treatment-effect heterogeneity.
Results
In the high-risk stage II subgroup, compared to the non-recipients, the hazard ratios (HR) of overall mortality for recipients were 0.83 (P = 0.001). The subdistribution hazard ratio (SHR) of CCSD for receipts was 1.22 (P = 0.021). The SHR of non-CCSD was 0.63 (P < 0.001). In the stage III subgroup, compared to non-recipients, the HR of the overall mortality for the recipients was 0.62 (P < 0.001). The SHR of CCSD was 0.77 (P < 0.001). The SHR of non-CCSD was 0.58 (P < 0.001). The chemotherapy efficacy differed significantly by risk score of non-CCSD (non-CCSD-RS) (P < 0.001). Recipients with high non-CCSD-RS had a rate of CCSD comparative to that of non-recipients (SHR 0.90, P = 0.150) in the stage III subgroup.
Conclusions
A survival analysis based on the overall mortality did not correctly interpret the effect of chemotherapy. Adjuvant chemotherapy did not provide an additional benefit to patients with high-risk stage II or patients with stage III at high risk of non-cancer death.






Similar content being viewed by others
References
Aapro MS, Kohne CH, Cohen HJ, Extermann M (2005) Never too old? Age should not be a barrier to enrollment in cancer clinical trials. Oncologist 10:198–204. https://doi.org/10.1634/theoncologist.10-3-198
Abraham A et al (2013) Adjuvant chemotherapy for stage III colon cancer in the oldest old: results beyond clinical guidelines. Cancer 119:395–403. https://doi.org/10.1002/cncr.27755
Ananda S et al (2008) Patient age and comorbidity are major determinants of adjuvant chemotherapy use for stage III colon cancer in routine clinical practice. J Clin Oncol 26:4516–4517. https://doi.org/10.1200/JCO.2008.18.7443 (author reply 4517–4518)
Andersen PK, Geskus RB, de Witte T, Putter H (2012) Competing risks in epidemiology: possibilities and pitfalls. Int J Epidemiol 41:861–870. https://doi.org/10.1093/ije/dyr213
Austin PC (2011) An Introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res 46:399–424. https://doi.org/10.1080/00273171.2011.568786
Bonetti M, Zahrieh D, Cole BF, Gelber RD (2009) A small sample study of the STEPP approach to assessing treatment-covariate interactions in survival data. Stat Med 28:1255–1268. https://doi.org/10.1002/sim.3524
Bradley CJ, Given CW, Dahman B, Fitzgerald TL (2008) Adjuvant chemotherapy after resection in elderly Medicare and Medicaid patients with colon cancer. Arch Intern Med 168:521–529. https://doi.org/10.1001/archinternmed.2007.82
Chagpar R, Xing Y, Chiang YJ, Feig BW, Chang GJ, You YN, Cormier JN (2012) Adherence to stage-specific treatment guidelines for patients with colon cancer. J Clin Oncol 30:972–979. https://doi.org/10.1200/JCO.2011.39.6937
de Glas NA et al (2016) Performing survival analyses in the presence of competing risks: a clinical example in older breast cancer patients. J Natl Cancer Inst. https://doi.org/10.1093/jnci/djv366
Erlichman C (1999) Efficacy of adjuvant fluorouracil and folinic acid in B2 colon cancer. International Multicentre Pooled Analysis of B2 Colon Cancer Trials (IMPACT B2. Investig J Clin Oncol 17:1356–1363
Fata F et al (2002) Efficacy and toxicity of adjuvant chemotherapy in elderly patients with colon carcinoma: a 10-year experience of the Geisinger. Med Center Cancer 94:1931–1938
Fine JP, Gray RJ (1999) A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94:496–509
Gill S et al (2004) Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: who benefits and by how much? J Clin Oncol 22:1797–1806. https://doi.org/10.1200/jco.2004.09.059
Haller DG, Catalano PJ, Macdonald JS, O’Rourke MA, Frontiera MS, Jackson DV, Mayer RJ (2005) Phase III study of fluorouracil, leucovorin, and levamisole in high-risk stage II and III colon cancer: final report of Intergroup 0089. J Clin Oncol 23:8671–8678. https://doi.org/10.1200/JCO.2004.00.5686
Han SS, Rivera GA, Tammemägi MC, Plevritis SK, Gomez SL, Cheng I, Wakelee HA (2017) Risk stratification for second primary lung cancer. J Clin Oncol 35(25):2893–2899. https://doi.org/10.1200/JCO.2017.72.4203
Keating NL et al (2008) Adjuvant chemotherapy for stage III colon cancer: do physicians agree about the importance of patient age and comorbidity? J Clin Oncol 26:2532–2537. https://doi.org/10.1200/JCO.2007.15.9434
Kim JH (2015) Chemotherapy for colorectal cancer in the elderly. World J Gastroenterol 21:5158–5166. https://doi.org/10.3748/wjg.v21.i17.5158
Kohne CH, Folprecht G, Goldberg RM, Mitry E, Rougier P (2008) Chemotherapy in elderly patients with colorectal cancer. Oncologist 13:390–402. https://doi.org/10.1634/theoncologist.2007-0043
Lee L, Cheung WY, Atkinson E, Krzyzanowska MK (2011) Impact of comorbidity on chemotherapy use and outcomes in solid tumors: a systematic review. J Clin Oncol 29:106–117. https://doi.org/10.1200/JCO.2010.31.3049
McCleary NJ et al (2013) Impact of age on the efficacy of newer adjuvant therapies in patients with stage II/III colon cancer: findings from the ACCENT database. J Clin Oncol 31:2600–2606. https://doi.org/10.1200/JCO.2013.49.6638
McCleary NJ, Dotan E, Browner I (2014) Refining the chemotherapy approach for older patients with colon cancer. J Clin Oncol 32:2570–2580. https://doi.org/10.1200/JCO.2014.55.1960
Merchant SJ, Nanji S, Brennan K, Karim S, Patel SV, Biagi JJ, Booth CM (2017) Management of stage III colon cancer in the elderly: practice patterns and outcomes in the general population. Cancer 123:2840–2849. https://doi.org/10.1002/cncr.30691
Muss HB, Biganzoli L, Sargent DJ, Aapro M (2007) Adjuvant therapy in the elderly: making the right decision. J Clin Oncol 25:1870–1875. https://doi.org/10.1200/JCO.2006.10.3457
Sanoff HK, Bleiberg H, Goldberg RM (2007) Managing older patients with colorectal cancer. J Clin Oncol 25:1891–1897. https://doi.org/10.1200/JCO.2006.10.1220
Sargent D et al (2009) Evidence for cure by adjuvant therapy in colon cancer: observations based on individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol 27:872–877. https://doi.org/10.1200/jco.2008.19.5362
Schrag D, Cramer LD, Bach PB, Begg CB (2001) Age and adjuvant chemotherapy use after surgery for stage III colon cancer. J Natl Cancer Inst 93:850–857
Scrucca L, Santucci A, Aversa F (2010) Regression modeling of competing risk using R: an in depth guide for clinicians. Bone Marrow Transplant 45:1388–1395. https://doi.org/10.1038/bmt.2009.359
Siegel RL, Miller KD, Jemal A (2017) Cancer statistics, 2017 CA: a cancer. J Clin 67:7–30. https://doi.org/10.3322/caac.21387
Wildiers H et al (2013) End points and trial design in geriatric oncology research: a joint European organisation for research and treatment of cancer–Alliance for Clinical Trials in Oncology–International Society Of Geriatric Oncology position article. J Clin Oncol 31:3711–3718. https://doi.org/10.1200/JCO.2013.49.6125
Yancik R (1997) Cancer burden in the aged: an epidemiologic and demographic overview. Cancer 80:1273–1283
Funding
The research was supported by (1) Key Projects in the National Science & Technology Pillar Program during the 12th 5-Year Plan Period (2014BAI09B07) and (2) National Key R&D Program of China (2017YFC0908200).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Author Dan Li declares that he has no conflict of interest. Author Chenhan Zhong declares that she has no conflict of interest. Author Xiujun Tang declares that she has no conflict of interest. Author Linzhen Yu declares that she has no conflict of interest. Author Kefeng Ding declares that he has no conflict of interest. Author Ying Yuan declares that she has no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
No ethics approval was sought for this study by authors as data used were the National Cancer Institute’s Surveillance, Epidemiology, and End Result (SEER) database, which is a publicly available de-identified population-based database.
Additional information
Dan Li and Chenhan Zhong are co-first authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, D., Zhong, C., Tang, X. et al. Competing nomograms help in the selection of elderly patients with colon cancer for adjuvant chemotherapy. J Cancer Res Clin Oncol 144, 909–923 (2018). https://doi.org/10.1007/s00432-018-2611-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-018-2611-y