Abstract
Background
Apatinib, a third-line or later treatment for advanced gastric cancer (aGC), was shown to improve overall survival and progression-free survival (PFS) compared with placebo in the phase III trial. Given the modest benefit with high costs, we further evaluated the cost-effectiveness of apatinib for patients with chemotherapy-refractory aGC.
Methods
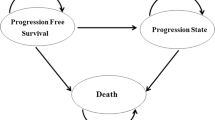
A Markov model was developed to simulate the disease process of aGC (PFS, progressive disease, and death) and estimate the incremental cost-effectiveness ratio (ICER) of apatinib to placebo. The health outcomes and utility scores were derived from the phase III trial and previously published sources, respectively. Total costs were calculated from the perspective of the Chinese health-care payer. Sensitivity analysis was used to explore model uncertainties.
Results
Treatment with apatinib was estimated to provide an incremental 0.09 quality-adjusted life years (QALYs) at an incremental cost of $8113.86 compared with placebo, which resulted in an ICER of $90,154.00 per QALY. Sensitivity analysis showed that across the wide variation of parameters, the ICER exceeded the willingness-to-pay threshold of $23,700.00 per QALY which was three times the Gross Domestic Product per Capita in China.
Conclusions
Apatinib is not a cost-effective option for patients with aGC who experienced failure of at least two lines chemotherapy in China. However, for its positive clinical value and subliminal demand, apatinib can provide a new therapeutic option.



Similar content being viewed by others
References
Ajani JA, Ilson DH, Daugherty K, Pazdur R, Lynch PM, Kelsen DP (1994) Activity of taxol in patients with squamous cell carcinoma and adenocarcinoma of the esophagus. J Natl Cancer Inst 86:1086–1091
Apatinib got CFDA approval (2015) http://www.xinyaohui.com/news/201502/05/5059.html. Accessed 27 July 2016
Bang YJ, Van Cutsem E, Feyereislova A et al (2010) Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376:687–697
ClinicalTrials.gov (2015) Apatinib in combination with S-1 as first-line treatment in patients with advanced gastric cancer. https://clinicaltrials.gov/ct2/show/NCT02525237?term=apatinib&rank=25. Accessed 27 July 2016
ClinicalTrials.gov (2016) Apatinib plus docetaxel versus docetaxel as second-line treatment in advanced gastric cancer (AHEAD-301). https://clinicaltrials.gov/ct2/show/NCT02596256?term=apatinib&rank=36. Accessed 27 July 2016
CNY Central Historical Parity Rate (2016) http://www.chinamoney.com.cn/english/bmkcpr/index.html?tab=2. Accessed 7 Oct 2016
Cunningham SC et al (2005) Survival after gastric adenocarcinoma resection: eighteen-year experience at a single institution. J Gastrointest Surg 9:718–725. doi:10.1016/j.gassur.2004.12.002
Ferlay J, Soerjomataram I, Dikshit R et al (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136:E359–E386
Fuchs CS, Tomasek J, Yong CJ et al (2014) Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 383:31–39
Goldstein DA, Ahmad BB, Chen Q et al (2015) Cost-effectiveness analysis of regorafenib for metastatic colorectal cancer. J Clin Oncol 33:3727–3732
Grothey A, Van Cutsem E, Sobrero A et al (2013) Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 381:303–312
He J, Wen F, Yin X et al (2013) Cost analysis of S1 and XELOX as adjuvant therapy for gastric cancer. Anticancer Drugs 24:754–758
Hironaka S, Ueda S, Yasui H et al (2013) Randomized, open-label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 trial. J Clin Oncol 31:4438–4444
Ilson DH (2016) Targeting the vascular endothelial growth factor pathway in gastric cancer: a hit or a miss? J Clin Oncol 34:1431–1432
Kamangar F, Dores GM, Anderson WF (2006) Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol 24:2137–2150
Lee J, Lim do H, Kim S et al (2012) Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. J Clin Oncol 30:268–273
Li J, Qin S, Xu J et al (2016) Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol 34:1448–1454
List of Chinese administrative divisions by GDP per capita (2016) https://en.wikipedia.org/wiki/List_of_Chinese_administrative_divisions_by_GDP_per_capita. Accessed 7 Oct 2016
Noh SH, Park SR, Yang HK et al (2014) Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol 15:1389–1396
Purmonen T, Martikainen JA, Soini EJ et al (2008) Economic evaluation of sunitinib malate in second-line treatment of metastatic renal cell carcinoma in Finland. Clin Ther 30:382–392
Ross P, Nicolson M, Cunningham D et al (2002) Prospective randomized trial comparing mitomycin, cisplatin, and protracted venous-infusion fluorouracil (PVI 5-FU) With epirubicin, cisplatin, and PVI 5-FU in advanced esophagogastric cancer. J Clin Oncol 20:1996–2004
Roviello G, Ravelli A, Polom K et al (2016) Apatinib: a novel receptor tyrosine kinase inhibitor for the treatment of gastric cancer. Cancer Lett 372:187–191
Sakuramoto S, Sasako M, Yamaguchi T et al (2007) Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 357:1810–1820
SEER Cancer Statistics Review (CSR), 1975–2013 (2016). http://seer.cancer.gov/csr/1975_2013/. Accessed 27 July 2016
Shiroiwa T, Fukuda T, Shimozuma K (2011) Cost-effectiveness analysis of trastuzumab to treat HER2-positive advanced gastric cancer based on the randomised ToGA trial. Br J Cancer 105:1273–1278
Sonnenberg FA, Beck JR (1993) Markov models in medical decision making: a practical guide. Med Decis Mak 13:322–338
Van Cutsem E, Moiseyenko VM, Tjulandin S et al (2006) Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol 24:4991–4997
WHO Guide to Generalized Cost-Effectiveness Analysis (2016) http://www.who.int/choice/cost-effectiveness/generalized/en/. Accessed 7 Oct 2016
Wilke H, Muro K, Van Cutsem E et al (2014) Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 15:1224–1235
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
H.-D. Chen and J. Zhou have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Chen, HD., Zhou, J., Wen, F. et al. Cost-effectiveness analysis of apatinib treatment for chemotherapy-refractory advanced gastric cancer. J Cancer Res Clin Oncol 143, 361–368 (2017). https://doi.org/10.1007/s00432-016-2296-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-016-2296-z