Abstract
Introduction
Non-adherence to anti-hormonal therapy is a major problem in gynecologic oncology. Reasons reported are side effects and lack of support. The aim of our study was an analysis of influence of experiences of patients with endocrine therapy and communication and information on this topic and their influence on adherence.
Methods
We developed a structured questionnaire which was tested in a pilot version and then programmed as online questionnaire and presented to patient members of self-help and breast cancer organizations.
Results
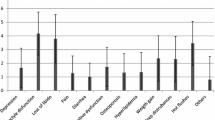
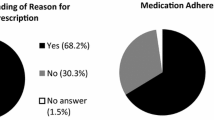
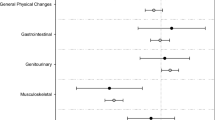
Patients only had received scarce information on endocrine therapy. Only 12.8 % stated that their questions were answered in detail, 43.2 % got no answers or only non-detailed answers. 76 % had side effects limiting functions of daily life. 60 % of physicians did not react on these side effects. There is a significant correlation between number and intensity of side effects and non-adherence or disruption of therapy (p = 0.029 and p < 0.01, respectively). Women who reported having received detailed answers to their questions also reported better adherence (p = 0.014).
Conclusion
In order to improve adherence, detailed information on side effects and answers in case of symptoms are most important. Physicians should not rely on presenting written information but should mainly engage in direct communication.
Similar content being viewed by others
References
Bell RJ, Fradkin P, Schwarz M, Davis SR (2013) Understanding discontinuation of oral adjuvant endocrine therapy by women with hormone receptor-positive invasive breast cancer nearly 4 years from diagnosis. Menopause 20(1):15–21
Chlebowski RT, Geller ML (2006) Adherence to endocrine therapy for breast cancer. Oncology 71(1–2):1–9
Cluze C, Rey D, Huiart L, BenDiane MK, Bouhnik AD, Berenger C, Carrieri MP, Giorgi R (2012) Adjuvant endocrine therapy with tamoxifen in young women with breast cancer: determinants of interruptions vary over time. Ann Oncol 23:882–890
Güth U, Huang DJ, Schötzau A, Zanetti-Dällenbach R, Holzgreve W, Bitzer J, Wight E (2008) Target and reality of adjuvant endocrine therapy in postmenopausal patients with invasive breast cancer. Br J Cancer 99(3):428–433
Güth U, Myrick ME, Kilic N, Eppenberger-Castori S, Schmid SM (2012) Compliance and persistence of endocrine adjuvant breast cancer therapy. Breast Cancer Res Treat 131(2):491–499
Hadjy P, Blettner M, Harbeck N, Jackisch C, Lück HJ, Windemuth-Kieselbach C, Zaun S, Kreienberg R (2013) The patient’s anastrozole compliance to therapy (PACT) program: a randomized, in-practice study in the impact of a standardized information program on persistence and compliance to adjuvant endocrine therapy in postmenopausal women with early breast cancer. Ann Oncol 24:505–512
Hershman DL, Kushi LH, Shao T, Buono D, Kershenbaum A, Tsai WY, Fehrenbacher L, Gomez SL, Miles S, Neugut AI (2010) Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol 28(27):4120–4128
Howell A, Cuzick J, Baum M, Buzdar A, Dowsett M, Forbes JF, Hoctin-Boes G, Houghton J, Locker GY, Tobias JS (2005) ATAC Trialists’ group: results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvanttreatment for breast cancer. Lancet 365:60–62
Huiart L, Dell’Aniello S, Suissa S (2011) Use of tamoxifen and aromatase inhibitors in a large population-based cohort of women with breast cancer. Br J Cancer 104(10):1558–1563
Land SR, Cronin WM, Wickerham DL, Costantino JP, Christian NJ, Klein WM, Ganz PA (2011) Cigarette smoking, obesity, physical activity, and alcohol use as predictors of chemoprevention adherence in the National Surgical Adjuvant Breast and Bowel Project P-1 Breast Cancer Prevention Trial. Cancer Prev Res (Phila) 4(9):1393–1400
Lash TL, Fox MP, Westrup JL, Fink AK, Silliman RA (2006) Adherence to tamoxifen over the five-year course. Breast Cancer Res Treat 99:215–220
Liu Y, Malin JL, Diamant AL, Thind A, Maly RC (2013) Adherence to adjuvant hormone therapy in low-income women with breast cancer: the role of provider-patient communication. Breast Cancer Res Treat 137:829–836
Murphy CC, Bartholomew LK, Carpentier MY, Bluethmann SM, Vernon SW (2012) Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: a systematic review. Breast Cancer Res Treat 134(2):459–478
Nekhlyudov L, Li L, Ross-Degnan D, Wagner AK (2011) Five-year patterns of adjuvant hormonal therapy use, persistence, and adherence among insured women with early-stage breast cancer. Breast Cancer Res Treat 130(2):681–689
Partridge AH, Wang PS, Winer EP, Avorn J (2003) Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol 21:602–606
Thurlimann B, Coates AS, Mouridsen H, Mauriac L, Forbes JF, Paridaens R, Castiglione-Gertsch M, Gelber RD, Rabaglio M, Smith I, Wardley A, Price KN, Goldhirsch A (2005) Breast international group (BIG) 1–98 collaborative group: a comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med 353:2747–2757
van de Water W, Bastiaannet E, Hille ET, Meershoek-Klein Kranenbarg EM, Putter H, Seynaeve CM, Paridaens R, de Craen AJ, Westendorp RG, Liefers GJ, van de Velde CJ (2012) Age-specific nonpersistence of endocrine therapy in postmenopausal patients diagnosed with hormone receptor-positive breast cancer: a TEAM study analysis. Oncologist 17(1):55–63
van Herk-Sukel MP, van de Poll-Franse LV, Voogd AC, Nieuwenhuijzen GA, Coebergh JW, Herings RM (2010) Half of breast cancer patients discontinue tamoxifen and any endocrine treatment before the end of the recommended treatment period of 5 years: a population-based analysis. Breast Cancer Res Treat 122(3):843–851
Wigertz A, Ahlgren J, Holmqvist M, Fornander T, Adolfsson J, Lindman H, Bergkvist L, Lambe M (2012) Adherence and discontinuation of adjuvant hormonal therapy in breast cancer patients: a population-based study. Breast Cancer Res Treat 133(1):367–373
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wuensch, P., Hahne, A., Haidinger, R. et al. Discontinuation and non-adherence to endocrine therapy in breast cancer patients: is lack of communication the decisive factor?. J Cancer Res Clin Oncol 141, 55–60 (2015). https://doi.org/10.1007/s00432-014-1779-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-014-1779-z