Abstract
Purpose
The efficacy and tolerability of bevacizumab every 2 or 4 weeks using the same dosage in combination with biweekly FOLFIRI were retrospectively evaluated in metastatic colorectal cancer (mCRC) patients in the first-line and second-line therapy.
Patients and methods
A total of 332 patients from six centers were evaluated. The patients had received biweekly FOLFIRI in combination with bevacizumab 5 mg/kg every 2 weeks or every 4 weeks schedule for various reasons in individual patients.
Results
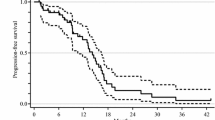
Approximately 70 % of all patients had 2-week treatment schedule. In the first-line therapy (n = 240), the overall response rate (ORR) was 34.1 % in 2-week and 36.3 % in 4-week groups. Median progression-free survival (PFS) was 8 months (95 %CI, 6.8–9.2) and 9 months (95 %CI, 6.6–11.4) (p = 0.074), and median overall survival (OS) was 22 months (95 %CI, 15.8–28.2) and 20 months (95 %CI, 8.1–31.9) (p = 0.612) in 2- and 4-week groups, respectively. One-year survival rate was 76.2 % for 2-week group and 73.2 % for 4-week group. In the second-line therapy (n = 92), the ORR was similar between the groups (24.5 vs 25.9 % in 2- and 4-week groups, respectively). Median PFS was 6 months (95 %CI, 4.7–7.3) and 11 months (95 %CI, 6.3–15.7) (p = 0.074), and median OS was 15 months (95 %CI, 9.6–20.4) and 17 months (95 %CI, 13.7–20.3) (p = 0.456) for 2-week and for 4-week groups, respectively. One-year survival rate was 61.3 % for 2-week and 71.3 % for 4-week groups. Toxicity profile was similar in 2- and 4-week groups and included neutropenia, febrile neutropenia, nausea and vomiting, diarrhea, mucositis, bleeding, hypertension, thromboembolism and fistulization.
Conclusion
Bevacizumab 5 mg/kg every 2 weeks or every 4 weeks in combination with biweekly FOLFIRI had similar efficacy and tolerability in mCRC. Because of the retrospective nature of our study, the data should be examined cautiously. However, our study clearly points out the need for determination of optimum biological dosing interval of bevacizumab in well-designed, prospective, randomized trials.




Similar content being viewed by others
References
Beer PM, Wong SJ, Hammad AM, Falk NS, O’Malley MR, Khan S (2006) Vitreous levels of unbound bevacizumab and unbound vascular endothelial growth factor in two patients. Retina 26:871–876
Bennouna J, Borg C, Delord JP, Husseini F, Trillet-Lenoir V, Faroux R, François E, Ychou M, Goldwasser F, Bouché O, Senellart H, Kraemer S, Douillard JY (2011) Bevacizumab combined with chemotherapy in the second-line treatment of metastatic colorectal cancer: results from the phase II BEVACOLOR study. Clin Colorectal Cancer. doi:10.1016/j.clcc.201105.002
Bergsland E, Dickler MN (2004) Maximizing the potential of bevacizumab in cancer treatment. Oncologist 9(Suppl 1):36–42
Borgström P, Gold DP, Hillan KJ, Ferrara N (1999) Importance of VEGF for breast cancer angiogenesis in vivo: implications from intravital microscopy of combination treatments with an anti-VEGF neutralizing monoclonal antibody and doxorubicin. Anticancer Res 19:4203–4214
Cobleigh MA, Langmuir VK, Sledge GW, Miller KD, Haney L, Novotny WF, Reimann JD, Vassel A (2003) A phase I/II dose-escalation trial of bevacizumab in previously treated metastatic breast cancer. Semin Oncol 30(Suppl 16):117–124
Folkman J (1990) What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst 82:4–6
Fuchs CS, Marshall J, Mitchell E, Wierzbicki R, Ganju V, Jeffery M, Schulz J, Richards D, Soufi-Mahjoubi R, Wang B, Barrueco J (2007) Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: results from the BICC-C Study. J Clin Oncol 25:4779–4786
Geraci MC (2004) National PBM drug monograph bevacizumab (avastin™). http://www.pbm.va.gov/Clinical%20Guidance/Drug%20Monographs/Bevacizumab.pdf. Accessed 05 October 2006
Gordon MS, Margolin K, Talpaz M, Sledge GW Jr, Holmgren E, Benjamin R, Stalter S, Shak S, Adelman D (2001) Phase I safety and pharmacokinetic study of recombinant human anti-vascular endothelial growth factor in patients with advanced cancer. J Clin Oncol 19:843–850
Grothey A, Sugrue MM, Purdie DM, Dong W, Sargent D, Hedrick E, Kozloff M (2008) Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: results from a large observational cohort study (BRiTE). J Clin Oncol 26:5326–5334
Jain RK (2001) Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med 7:987–989
Kabbinavar F, Hurwitz HI, Fehrenbacher L, Meropol NJ, Novotny WF, Lieberman G, Griffing S, Bergsland E (2003) Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol 21:60–65
Kaplan EL, Meier P (1958) Nonparametric estimation for incomplete observations. J Am Stat Assoc 53:457–481
Kwon HC, Oh SY, Lee S, Kim SH, Kim HJ (2007) Bevacizumab plus infusional 5-fluorouracil, leucovorin and irinotecan for advanced colorectal cancer that progressed after oxaliplatin and irinotecan chemotherapy: a pilot study. World J Gastroenterol 13:6231–6235
Moriwaki T, Bando H, Takashima A, Yamazaki K, Esaki T, Yamashita K, Fukunaga M, Miyake Y, Katsumata K, Kato S, Satoh T, Ozeki M, Baba E, Yoshida S, Boku N, Hyodo I (2011) Bevacizumab in combination with irinotecan, 5-fluorouracil, and leucovorin (FOLFIRI) in patients with metastatic colorectal cancer who were previously treated with oxaliplatin-containing regimens: a multicenter observational cohort study (TCTG 2nd-BV study). Med Oncol. doi:10.1007/s12032-011-0151-2
National Cancer Institute Common Toxicity Criteria (NCI-CTC) v3.0. 2006. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf Accessed 16 November 2007
Odabas H, Ozdemir N, Isik M, Abali H, Oksuzoglu B, Kos T, Civelek B, Babacan AN, Dogan U, Zengin N (2011) Safety and efficacy of FOLFIRI-bevacizumab for metastatic colorectal carcinoma as second line treatment. J BUON 16:460–463
Rak JW, St Croix BD, Kerbel RS (1995) Consequences of angiogenesis for tumor progression, metastasis and cancer therapy. Anticancer Drugs 6:3–18
Sher AF, Chu D, Wu S (2009) Risk of bleeding in cancer patients treated with the angiogenesis inhibitor bevacizumab: a meta-analysis. J Clin Oncol 27(Suppl; abstr 9584):15s
Sobrero A, Ackland S, Clarke S, Perez-Carrión R, Chiara S, Gapski J, Mainwaring P, Langer B, Young S, AVIRI Trial investigators (2009) Phase IV study of bevacizumab in combination with infusional fluorouracil, leucovorin and irinotecan (FOLFIRI) in first-line metastatic colorectal cancer. Oncology 77:113–119
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Van Cutsem E, Rivera F, Berry S, Kretzschmar A, Michael M, DiBartolomeo M, Mazier MA, Canon JL, Georgoulias V, Peeters M, Bridgewater J, Cunningham D; First BEAT investigators (2009) Safety and efficacy of first-line bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyrimidines in metastatic colorectal cancer: the BEAT study. Ann Oncol 20:1842–1847
Yildiz R, Buyukberber S, Uner A, Yamac D, Coskun U, Kaya AO, Ozturk B, Yaman E, Benekli M (2010) Bevacizumab plus irinotecan-based therapy in metastatic colorectal cancer patients previously treated with oxaliplatin-based regimens. Cancer Invest 28:33–37
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was conducted for Anatolian Society of Medical Oncology (ASMO).
Rights and permissions
About this article
Cite this article
Yildiz, R., Benekli, M., Ozkan, M. et al. Bevacizumab every 4 weeks is as effective as every 2 weeks in combination with biweekly FOLFIRI in metastatic colorectal cancer. J Cancer Res Clin Oncol 138, 1845–1852 (2012). https://doi.org/10.1007/s00432-012-1264-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-012-1264-5