Abstract
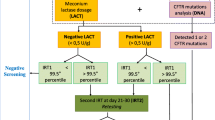
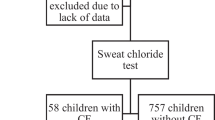
Newborn screening for cystic fibrosis (CF), a chronic progressive disease affecting mucus viscosity, has been beneficial in both improving life expectancy and the quality of life for individuals with CF. In New York State from 2007 to 2012 screening for CF involved measuring immunoreactive trypsinogen (IRT) levels in dried blood spots from newborns using the IMMUCHEM™ Blood Spot Trypsin-MW ELISA kit. Any specimen in the top 5 % IRT level underwent DNA analysis using the InPlex® CF Molecular Test. Of the 1.48 million newborns screened during the 6-year time period, 7631 babies were referred for follow-up. CF was confirmed in 251 cases, and 94 cases were diagnosed with CF transmembrane conductance regulated-related metabolic syndrome or possible CF. Nine reports of false negatives were made to the program. Variation in daily average IRT was observed depending on the season (4–6 ng/ml) and kit lot (<3 ng/ml), supporting the use of a floating cutoff. The screening method had a sensitivity of 96.5 %, specificity of 99.6 %, positive predictive value of 4.5 %, and negative predictive value of 99.5 %.
Conclusion: Considerations for CF screening algorithms should include IRT variations resulting from age at specimen collection, sex, race/ethnicity, season, and manufacturer kit lots.
What is Known: • Measuring IRT level in dried blood spots is the first-tier screen for CF • Current algorithms for CF screening lead to substantial false-positive referral rates |
What is New: • IRT values were affected by age of infant when specimen is collected, race/ethnicity and sex of infant, and changes in seasons and manufacturer kit lots • The prevalence of CF in NYS is 1 in 4200 with the highest prevalence in White infants (1 in 2600) and the lowest in Black infants (1 in 15,400) |


Similar content being viewed by others
Abbreviations
- ACMG:
-
American College of Medical Genetics
- CBAVD:
-
Congenital bilateral absence of the vas deferens
- CF:
-
Cystic fibrosis
- CFTR :
-
Cystic fibrosis transmembrane conductance regulator
- CRMS:
-
CFTR-related metabolic syndrome
- DOB:
-
Day of birth
- IRT:
-
Immunoreactive trypsinogen
- NBS:
-
Newborn screening
- NGS:
-
Next-generation sequencing
- NICU:
-
Neonatal intensive care unit
- NPV:
-
Negative predictive value
- NYS:
-
New York State
- MI:
-
Meconium ileus
- PAP:
-
Pancreatitis-associated protein
- PPV:
-
Positive predictive value
References
Anzai C, Morokawa N, Okada H, Kamidono S, Eto Y (2003) CFTR Gene mutations in Japanese individuals with congenital bilateral absence of the vas deferens. J Cyst Fibros 2:14–18
Baker MW, Atkins AE, Cordovado SK, Hendrix M, Earley MC, Farrell PM (2015) Improving newborn screening for cystic fibrosis using next-generation sequencing technology: a technical feasibility study. Genet Med 1–8
Balfour-Lynn IM (2008) Cystic fibrosis papers of the year 2007. J Roy Soc Med 101(Suppl):S10–S14
Barben J, Gallati S, Fingerhut R, Schoeni MH, Baumgartner MR, Torresani T (2012) Retrospective analysis of stored dried blood spots from children with cystic fibrosis and matched controls to assess the performance of a proposed newborn screening protocol in Switzerland. J Cyst Fibros 11:332–336
Barthellemy S, Maurin N, Roussey M, Férec C, Murolo S, Berthézène P, Iovanna JL, Dagorn JC, Sarles J (2001) Evaluation of 47,213 infants in neonatal screening for cystic fibrosis, using pancreatitis-associated protein and immunoreactive trypsinogen assays. Arch Pediatr 8:275–281
Borrajo GJC (2007) Newborn screening in Latin America at the beginning of the 21st century. J Inherit Metab Dis 30:466–481
Bauça JM, Morell-Garcia D, Vila M, Pérez G, Heine-Suñer D, Figuerola J (2015) Assessing the improvements in the newborn screening strategy for cystic fibrosis in the Balearic Islands. J Clin Biochem 48:419–424
Calvin J, Hogg SL, McShane D, McAuley SA, Iles R, Ross-Russell R, MacLean FM, Heeley ME, AF Heeley AF, Written on behalf of the Norfolk, Suffolk and Cambridgeshire Paediatric Cystic Fibrosis Network (2012) Thirty-years of screening for cystic fibrosis in East Anglia. Arch Dis Child 97:1043–1047
Castellani C, Cuppens H, Macek M Jr, Cassiman JJ, Kerem E, Durie P, Tullis E, Assael BM, Bombieri C, Brown A, Casals T, Claustres M, Cutting GR, Dequeker E, Dodge J, Doull I, Farrell P, Ferec C, Girodon E, Johannesson M, Kerem B, Knowles M, Munck A, Pignatti PF, Radojkovic D, Rizzotti P, Schwarz M, Stuhrmann M, Tzetis M, Zielenski J, Elborn JS (2008) Consensus on the use and interpretation of cystic fibrosis mutation analysis in clinical practice. J Cyst Fibros 7:179–196
Castellani C, Picci L, Scarpa M, Dechecchi MC, Zanolla L, Assael BM, Zacchello F (2005) Cystic fibrosis carriers have higher neonatal immunoreactive trypsinogen values than non-carriers. Am J Med Genet Part A 135:142–144
Castellani C, Picci L, Tamanini A, Girardi P, Rizzotti P, Assael BM (2009) Association between carrier screening and incidence of cystic fibrosis. J Am Med Assoc 302:2573–2579
Castellani C, Picci L, Tridello G, Casati E, Tamanini A, Bartoloni L, Scarpa M, Assael BM (2015) Cystic fibrosis carrier screening effects on birth prevalence and newborn screening. Genet Med In Press.
Comeau AM, Parad RB, Dorkin HL, Dovey M, Gerstle R, Haver K, Lapey A, O’Sullivan BP, Waltz DA, Zwerdling RG, Eaton RB (2004) Population-based newborn screening for genetic disorders when multiple mutation DNA testing is incorporated: a cystic fibrosis newborn screening model demonstrating increased sensitivity but more carrier detections. Pediatrics 113:1573–1581
Cornel MC, Gille JJ, Loeber JG, Vernooij-van Langen AM, Dankert-Roelse J, Bolhuis PA (2012) Improving test properties for neonatal cystic fibrosis screening in the Netherlands before the nationwide start by May 1st 2011. J Inherit Metab Dis 35:635–640
Cortés E, Roldán AM, Palazón-Bru A, Rizo-Baeza MM, Manero H, Gil-Guillén VF (2014) Differences in immunoreactive trypsin values between type of feeding and ethnicity in neonatal cystic fibrosis screening: a cross-sectional study. Orphanet J Rare Dis 9:166–172
Crossley JR, Elliott RB, Smith PA (1979) Dried-blood spot screening for cystic fibrosis in the newborn. Lancet 2:472–474
Davidson AG, Wong LT, Kirby LT, Applegarth DA (1984) Immunoreactive trypsin in cystic fibrosis. J Pediatr Gastroenterol Nutr 3:S79–S88
Davies JC (2002) Pseudomonas aeruginosa in cystic fibrosis: pathogenesis and persistence. Paediatr Respir Rev 3:128–134
Davis PB (2006) Cystic fibrosis since 1938. Am J Respir Crit Care Med 173:475–482
Dequeker E, Stuhrmann M, Morris MA, Casals T, Castellani C, Claustres M, Cuppens H, des Georges M, Ferec C, Macek M, Pignatti P-F, Scheffer H, Schwartz M, Witt M, Schwarz M, Girodon E (2008) Best practice guidelines for molecular genetic diagnosis of cystic fibrosis and CFTR-related disorders—updated European recommendations. Eur J Hum Genet 17:51–65
Dijk FN, Fitzgerald DA (2012) The impact of newborn screening and earlier intervention on the clinical course of cystic fibrosis. Paediatr Respir Rev 13:220–225
Farrell PM, Kosorok MR, Laxova A, Shen G, Koscik RE, Bruns WT, Splaingard M, Mischler EH (1997) Nutritional benefits of neonatal screening for cystic fibrosis. Wisconsin cystic fibrosis neonatal screening study group. N Engl J Med 337:963–969
Farrell PM, Lai HJ, Kosorok MR, Laxova A, Green CG, Collins J, Hoffman G, Laessig R, Rock MJ, Splaingard ML (2005) Evidence on improved outcomes with early diagnosis of cystic fibrosis through neonatal screening: enough is enough! J Pediatr 147:S30–S36
Farrell PM, Rosenstein BJ, White TB, Accurso FJ, Castellani C, Cutting GR, Durie PR, Legrys VA, Massie J, Parad RB, Rock MJ, Campbell PW 3rd, Cystic Fibrosis Foundation (2008) Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation consensus report. J Pediatr 153:S4–S14
Gibson LE, Cooke RE (1959) A test for concentration of electrolytes in sweat in cystic fibrosis of the pancreas utilizing pilocarpine by iontophoresis. Pediatrics 23:545–549
Giusti R, Badgwell A, Iglesias AD, and the New York State Cystic Fibrosis Newborn Screening Consortium State (2007) New York State Cystic Fibrosis Consortium: the first 2.5 years of experience with cystic fibrosis newborn screening in an ethnically diverse population. Pediatrics 119:e460–e467
Grosse SD, Rosenfeld M, Devine OJ, Lai HJ, Farrell PM (2006) Potential impact of newborn screening for cystic fibrosis on child survival: a systematic review and analysis. J Pediatr 149:362–366
Hale JE, Parad RB, Comeau AM (2008) Newborn screening showing decreasing incidence of cystic fibrosis. N Engl J Med 358:973–974
Heijerman H (2005) Infection and inflammation in cystic fibrosis: a short review. J Cyst Fibros 4(Suppl 2):3–5
Iovanna JL, Férec C, Sarles J, Dagorn JC (1994) The pancreatitis-associated protein (PAP). A new candidate for neonatal screening of cystic fibrosis. C R Acad Sci III 317:561–564
Kay DM, Langfelder-Schwind E, DeCelie-Germana J, Sharp JK, Maloney B, Tavakoli NP, Saavedra-Matiz CA, Krein LM, the New York State Cystic Fibrosis Newborn Screening Consortium, Caggana M, Kier C. Utility of a very high IRT/no mutation referral category in cystic fibrosis newborn screening: analysis of 2.4 million New York babies. Ped Pulmonol 50:771–780
Kerem B, Rommens JM, Buchanan JA, Markiewicz D, Cox TK, Chakravarti A, Buchwald M, Tsui LC (1989) Identification of the cystic fibrosis gene: genetic analysis. Science 245:1073–1080
Kiesewetter S, Macek M Jr, Davis C, Curristin SM, Chu C-S, Graham C, Shrimpton AE, Cashman SM, Tsui LC, Mickle J, Amos J, Highsmith WE, Shuber A, Witt DR, Crystal RG, Cutting GR (1993) A mutation in CFTR produces different phenotypes depending on chromosomal background. Nat Genet 5:274–278
Kloosterboer M, Hoffman G, Rock M, Gershan W, Laxova A, Li Z, Farrell PM (2009) Clarification of laboratory and clinical variables that influence cystic fibrosis newborn screening with initial analysis of immunoreactive trypsinogen. Pediatrics 123:e338–e346
Korzeniewski SJ, Young WI, Hawkins HC, Cavanagh K, Nasr SZ, Langbo C, Teneyck KR, Grosse SD, Kleyn M, Grigorescu V (2011) Variation in immunoreactive trypsinogen concentrations among Michigan newborns and implications for cystic fibrosis newborn screening. Pediatr Pulmonol 46:125–130
Lai HC, Kosorok MR, Sondel SA, Chen ST, FitzSimmons SC, Green CG, Shen G, Walker S, Farrell PM (1998) Growth status in children with cystic fibrosis based on the National Cystic Fibrosis Patient Registry Data: evaluation of various criteria used to identify malnutrition. J Pediatr 132:478–485
Laroche D, Travert G (1991) Abnormal frequency of ΔF508 mutation in neonatal transitory hypertrypsinaemia. Lancet 337:355
Lecoq I, Brouard J, Laroche D, Ferec C, Travert G (1999) Blood immunoreactive trypsinogen concentrations are genetically determined in healthy and cystic fibrosis newborns. Acta Paediatr 88:338–341
LeGrys VA, Yankaskas JR, Quittell LM, Marshall BC, Mogayzel PJ (2007) Diagnostic sweat testing: the Cystic Fibrosis Foundation guidelines. J Pediatr 151:85–89
Lim MT, Wallis C, Price JF, Carr SB, Chavasse RJ, Shankar A, Seddon P, Balfour-Lynn IM (2014) Diagnosis of cystic fibrosis in London and South East England before and after the introduction of newborn screening. Arch Dis Child 99:197–202
Lobo J, Rojas-Balcazar JM, Noone PG (2012) Recent advances in cystic fibrosis. Clin Chest Med 33:307–328
Massie J, Curnow L, Tzanakos N, Francis I, Robertson CF (2006) Markedly elevated neonatal immunoreactive trypsinogen levels in the absence of cystic fibrosis gene mutations is not an indication for further testing. Arch Dis Child 91:222–225
Monaghan KG, Highsmith WE, Amos J, Pratt VM, Roa B, Friez M, Pike-Buchanan LL, Buyse IM, Redman JB, Strom CM, Young AL, Sun W (2004) Genotype-phenotype correlation and frequency of the 3199del6 cystic fibrosis mutation among I148T carriers: results from a collaborative study. Genet Med 6:421–425
Paracchini V, Seia M, Raimondi S, Costantino L, Capasso P, Porcaro L, Colombo C, Coviello DA, Mariani T, Manzoni E, Sangiovanni M, Corbetta C (2012) Cystic fibrosis newborn screening: distribution of blood immunoreactive trypsinogen concentrations in hypertrypsinemic neonates. J Inherit Metab Dis Rep 4:17–23
Pollak A, Kasper DC (2014) Austrian Newborn Screening Program: a perspective of five decades. J Perinat Med 42:151–158
Prach L, Koepke R, Kharrazi M, Keiles S, Salinas DB, Reyes MC, Pian M, Opsimos H, Otsuka KN, Hardy KA, Milla CE, Zirbes JM, Chipps B, O’Bra S, Saeed MM, Sudhakar R, Lehto S, Nielson D, Shay GF, Seastrand M, Jhawar S, Nickerson B, Landon C, Thompson A, Nussbaum E, Chin T, Wojtczak H, California Cystic Fibrosis Newborn Screening Consortium (2013) Novel CFTR variants identified during the first 3 years of cystic fibrosis newborn screening in California. J Mol Diagn 15:710–722
Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL, Drumm ML, Iannuzzi MC, Collins FS, Tsui LC (1989) Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 245:1066–1073
Rock MJ, Hoffman G, Laessig RH, Kopish GJ, Litsheim TJ, Farrell PM (2005) Newborn screening for cystic fibrosis in Wisconsin: nine-year experience with routine trypsinogen/DNA testing. J Pediatr 147:S73–S77
Rock MJ, Mischler EH, Farrell PM, Bruns WT, Hassemer DJ, Laessig RH (1989) Immunoreactive trypsinogen screening for cystic fibrosis: characterization of infants with a false positive screening test. Pediatr Pulmonol 6:42–48
Rock MJ, Mischler EH, Farrell PM, Wei LJ, Bruns WT, Hassemer DJ, Laessig RH (1990) Newborn screening for cystic fibrosis is complicated by age-related decline in immunoreactive trypsinogen levels. Pediatrics 85:1001–1007
Rohlfs EM, Zhou Z, Sugarman EA, Heim RA (2002) The I148T CFTR allele occurs on multiple haplotypes: a complex allele is associated with cystic fibrosis. Genet Med 4:319–323
Rommens JM, Iannuzzi MC, Kerem B, Drumm ML, Melmer G, Dean M, Rozmahel R, Cole JL, Kennedy D, Hidaka N, Zsiga M, Riordan JR, Tsui LC (1989) Identification of the cystic fibrosis gene: chromosome walking and jumping. Science 245:1059–1065
Rosenstein BJ, Cutting GR (1998) The diagnosis of cystic fibrosis: a consensus statement. Cystic Fibrosis Foundation consensus panel. J Pediatr 132:589–595
Rusakow LS, Abman SH, Sokol RJ, Seltzer W, Hammond KB, Accurso FJ (1993) Immunoreactive trypsinogen levels in infants with cystic fibrosis complicated by meconium ileus. Screening 2:13–17
Sands D, Zybert K, Mierzejewska E, Ołtarzewski M (2015) Diagnosing cystic fibrosis in newborn screening in Poland—15 years of experience. Dev Period Med 19:16–24
Sarles J, Berthézène P, Le Louarn C, Somma C, Perini JM, Catheline M, Mirallié S, Luzet K, Roussey M, Farriaux JP, Berthelot J, Dagorn JC (2005) Combining immunoreactive trypsinogen and pancreatitis-associated protein assays, a method of newborn screening for cystic fibrosis that avoids DNA analysis. J Pediatr 147:302–305
Sarles J, Giorgi R, Berthézène P, Munck A, Cheillan D, Dagorn JC, Roussey M (2014) Neonatal screening for cystic fibrosis: comparing the performances of IRT/DNA and IRT/PAP. J Cyst Fibros 13:384–390
Scotet V, Duguépéroux I, Saliou P, Rault G, Roussey M, Audrézet MP, Férec C (2012) Evidence for decline in the incidence of cystic fibrosis: a 35-year observational study in Brittany, France. Orphanet J Rare Dis 7:14–20
Sobczyńska-Tomaszewska A, Ołtarzewski M, Czerska K, Wertheim-Tysarowska K, Sands D, Walkowiak J, Bal J, Mazurczak T (2013) Newborn screening for cystic fibrosis: Polish 4 years’ experience with CFTR sequencing strategy. Eur J Hum Genet 21:391–396
Sommerburg O, Hammermann J, Lindner M, Stahl M, Muckenthaler M, Kohlmueller D, Happich M, Kulozik AE, Stopsack M, Gahr M, Hoffmann GF, Mall MA (2015) Five years of experience with biochemical cystic fibrosis newborn screening based on IRT/PAP in Germany. Pediatr Pulmonol 50:655–664
Sontag MK, Corey M, Hokanson JE, Marshall JA, Sommer SS, Zerbe GO, Accurso FJ (2006) Genetic and physiologic correlates of longitudinal immunoreactive trypsinogen decline in infants with cystic fibrosis identified through newborn screening. J Pediatr 149:650–657
Sontag MK, Hammond KB, Zielenski J, Wagener JS, Accurso FJ (2005) Two-tiered immunoreactive trypsinogen-based newborn screening for cystic fibrosis in Colorado: screening efficacy and diagnostic outcomes. J Pediatr 147(Suppl):S83–S88
Southern KW (2007) Cystic fibrosis and formes frustes of CFTR-related disease. Respiration 74:241–251
Stern RC (1997) The diagnosis of cystic fibrosis. N Engl J Med 336:487–491
Therrell BL, Hannon WH, Hoffman G, Ojodu J, Farrell PM (2012) Immunoreactive trypsinogen (IRT) as a biomarker for cystic fibrosis: challenges in newborn dried blood spot screening. Mol Genet Metab 106:1–6
Torresani T, Fingerhut R, Rueegg CS, Gallati S, Kuehni CE, Baumgartner MR, Barben J, Swiss CF Screening Group (2013) Newborn screening for cystic fibrosis in Switzerland—consequences after analysis of a 4 months pilot study. J Cyst Fibros 12:667–674
VanDevanter DR, Pasta DJ, Konstan MW (2014) Improvements in lung function and height among cohorts of 6-year-olds with cystic fibrosis from 1994 to 2012. J Pediatr 165:1091–1097
Watson MS, Mann MY, Lloyd-Puryear MA, Rinaldo P, Howell RR (2006) Executive summary. Genet Med 8:1S–11S
Xu WM, Shi QX, Chen WY, Zhou CX, Ni Y, Rowlands DK, Yi Liu G, Zhu H, Ma ZG, Wang XF, Chen ZH, Zhou SC, Dong HS, Zhang XH, Chung YW, Yuan YY, Yang WX, Chan HC (2007) Cystic fibrosis transmembrane conductance regulator is vital to sperm fertilizing capacity and male fertility. Proc Natl Acad Sci U S A 104:9816–9821
Acknowledgments
The authors wish to thank Dr. Andrew Reilly for assistance with statistical analysis, the Newborn Screening Follow-up group, especially David Render and Amy McGeoch, for referring and closing cases, the NYS CF Specialty Care Centers and NYS CF Consortium for providing diagnostic information on referred cases, and Bryan Laplante for assistance with newborn screening data.
Compliance with ethical standards
ᅟ
Conflict of interest
The authors declare that they have no conflicts of interest, financially or otherwise.
The manuscript is a retrospective case report that does not require ethics committee approval at our institution (Wadsworth Center, NYSDOH). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Authors contributions
DMK, BV, and CAS were involved in analysis and interpretation of data and revising the manuscript. BM, RH, MP, LD, RM, EM, and LM were involved in acquisition of data and revising the manuscript. MC was involved in design of the work, interpreting data, and revising the manuscript. NPT was involved in design of the work, analysis and interpreting of data, and drafting the work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Beat Steinmann
Denise M. Kay and Breanne Maloney contributed equally to this work.
Rights and permissions
About this article
Cite this article
Kay, D.M., Maloney, B., Hamel, R. et al. Screening for cystic fibrosis in New York State: considerations for algorithm improvements. Eur J Pediatr 175, 181–193 (2016). https://doi.org/10.1007/s00431-015-2616-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-015-2616-3