Abstract
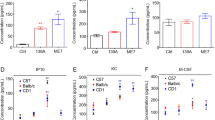
Activation of complement system in central nervous system (CNS) of the patients suffering from prion diseases or animal models infected with prion agents experimentally is reported repeatedly, but which pathways are involved in the complement system during prion infection is not well documented. Here, we evaluated the level of complement factor B (CFB), which is the key factor that triggers alterative pathway (AP) of complement in the brain tissues of scrapie-infected mice with various methodologies. We found that the levels of mRNA and protein of CFB significantly increased in the brain tissues of scrapie-infected mice. Morphologically, the increased CFB-specific signal overlapped with the elevated C3 signal in brain sections of scrapie-infected mice, meanwhile overlapped with damaged neurons and activated microglia, but not with the proliferative astrocytes. Additionally, the level of complement factor P (CFP), the key positive regulator of AP, also increased remarkably in the brain tissues of infected mice. The transcriptional levels of CD55 and CD46, two negative regulators of AP, decreased without significance in brain tissues of scrapie-infected mice at the terminal stage. However, the mRNA and protein levels of CFH, another negative regulator of AP, increased. Through the dynamic analyses of the expressions of CFB, CFP, and CFH in brain sections of 139A-infected mice, which were collected at different time-points during incubation period, illustrated time-dependent increase levels of each factor during the incubation period of scrapie infection. Taken together, our data here demonstrate that the AP of complement cascade is activated in the CNS microenvironment during prion infection.







Similar content being viewed by others
References
Prusiner SB (1998) Prions. Proc Natl Acad Sci U S A 95(23):13363–13383
Prusiner SB, Scott MR, DeArmond SJ, Cohen FE (1998) Prion protein biology. Cell 93(3):337–348
Will RG, Ironside JW, Zeidler M, Cousens SN, Estibeiro K, Alperovitch A, Poser S, Pocchiari M, Hofman A, Smith PG (1996) A new variant of Creutzfeldt-Jakob disease in the UK. Lancet 347(9006):921–925
Yanamadala V, Friedlander RM (2010) Complement in neuroprotection and neurodegeneration. Trends Mol Med 16(2):69–76
Veerhuis R, Nielsen HM, Tenner AJ (2011) Complement in the brain. Mol Immunol 48(14):1592–1603
Bonifati DM, Kishore U (2007) Role of complement in neurodegeneration and neuroinflammation. Mol Immunol 44(5):999–1010
Ishii T, Haga S, Yagishita S, Tateishi J (1984) The presence of complements in amyloid plaques of Creutzfeldt-Jakob disease and Gerstmann–Straussler–Scheinker disease. Appl Pathol 2(6):370–379
Klein MA, Kaeser PS, Schwarz P, Weyd H, Xenarios I, Zinkernagel RM, Carroll MC, Verbeek JS, Botto M, Walport MJ, Molina H, Kalinke U, Acha-Orbea H, Aguzzi A (2001) Complement facilitates early prion pathogenesis. Nat Med 7(4):488–492
Lv Y, Chen C, Zhang BY, Xiao K, Wang J, Chen LN, Sun J, Gao C, Shi Q, Dong XP (2014) Remarkable Activation of the Complement System and Aberrant Neuronal Localization of the Membrane Attack Complex in the Brain Tissues of Scrapie-Infected Rodents. Mol Neurobiol 52(3):1165–1179
Chen C, Lv Y, Shi Q, Zhou W, Xiao K, Sun J, Yang XD, Dong XP (2016) Low activity of complement in the cerebrospinal fluid of the patients with various prion diseases. Infectious diseases of poverty 5:35
Chen C, Xiao D, Zhou W, Shi Q, Zhang HF, Zhang J, Tian C, Zhang JZ, Dong XP (2014) Global Protein Differential Expression Profiling of Cerebrospinal Fluid Samples Pooled from Chinese Sporadic CJD and non-CJD Patients. Mol Neurobiol 49(1):290–302
Shi Q, Zhang BY, Gao C, Zhang J, Jiang HY, Chen C, Han J, Dong XP (2012) Mouse-adapted scrapie strains 139A and ME7 overcome species barrier to induce experimental scrapie in hamsters and changed their pathogenic features. Virol J 9:63
Zhang J, Chen L, Zhang BY, Han J, Xiao XL, Tian HY, Li BL, Gao C, Gao JM, Zhou XB, Ma GP, Liu Y, Xu CM, Dong XP (2004) Comparison study on clinical and neuropathological characteristics of hamsters inoculated with scrapie strain 263 K in different challenging pathways. Biomed Environ Sci 17(1):65–78
Chen C, Lv Y, Zhang BY, Zhang J, Shi Q, Wang J, Tian C, Gao C, Xiao K, Ren K, Zhou W, Dong XP (2014) Apparent Reduction of ADAM10 in Scrapie-Infected Cultured Cells and in the Brains of Scrapie-Infected Rodents. Mol Neurobiol 50(3):875–887
Gao JM, Gao C, Han J, Zhou XB, Xiao XL, Zhang J, Chen L, Zhang BY, Hong T, Dong XP (2004) Dynamic analyses of PrP and PrP(Sc) in brain tissues of golden hamsters infected with scrapie strain 263 K revealed various PrP forms. Biomed Environ Sci 17(1):8–20
Kovacs GG, Gasque P, Strobel T, Lindeck-Pozza E, Strohschneider M, Ironside JW, Budka H, Guentchev M (2004) Complement activation in human prion disease. Neurobiol Dis 15(1):21–28
Mabbott NA (2004) The complement system in prion diseases. Curr Opin Immunol 16(5):587–593
Narni-Mancinelli E, Gauthier L, Baratin M, Guia S, Fenis A, Deghmane AE, Rossi B, Fourquet P, Escaliere B, Kerdiles YM, Ugolini S, Taha MK, Vivier E (2017) Complement factor P is a ligand for the natural killer cell-activating receptor NKp46. Sci Immunol 2 (10): eaam9628
Yamada T, McGeer PL, McGeer EG (1992) Lewy bodies in Parkinson’s disease are recognized by antibodies to complement proteins. Acta Neuropathol 84(1):100–104
Singhrao SK, Neal JW, Morgan BP, Gasque P (1999) Increased complement biosynthesis by microglia and complement activation on neurons in Huntington’s disease. Exp Neurol 159(2):362–376
Yasojima K, Schwab C, McGeer EG, McGeer PL (1999) Up-regulated production and activation of the complement system in Alzheimer’s disease brain. Am J Pathol 154(3):927–936
Shi Q, Chen LN, Zhang BY, Xiao K, Zhou W, Chen C, Zhang XM, Tian C, Gao C, Wang J, Han J, Dong XP (2015) Proteomics analyses for the global proteins in the brain tissues of different human prion diseases. Mol Cell Proteomics 14(4):854–869
Gasque P, Dean YD, McGreal EP, VanBeek J, Morgan BP (2000) Complement components of the innate immune system in health and disease in the CNS. Immunopharmacology 49(1–2):171–186
Brodbeck WG, Kuttner-Kondo L, Mold C, Medof ME (2000) Structure/function studies of human decay-accelerating factor. Immunology 101(1):104–111
Liszewski MK, Farries TC, Lublin DM, Rooney IA, Atkinson JP (1996) Control of the complement system. Adv Immunol 61:201–283
Sjoberg A, Onnerfjord P, Morgelin M, Heinegard D, Blom AM (2005) The extracellular matrix and inflammation: fibromodulin activates the classical pathway of complement by directly binding C1q. J Biol Chem 280(37):32301–32308
Sjoberg AP, Trouw LA, Clark SJ, Sjolander J, Heinegard D, Sim RB, Day AJ, Blom AM (2007) The factor H variant associated with age-related macular degeneration (His-384) and the non-disease-associated form bind differentially to C-reactive protein, fibromodulin, DNA, and necrotic cells. J Biol Chem 282(15):10894–10900
Sjoberg AP, Manderson GA, Morgelin M, Day AJ, Heinegard D, Blom AM (2009) Short leucine-rich glycoproteins of the extracellular matrix display diverse patterns of complement interaction and activation. Mol Immunol 46(5):830–839
Perkins SJ, Nan R, Li K, Khan S, Miller A (2012) Complement factor H-ligand interactions: self-association, multivalency and dissociation constants. Immunobiology 217(2):281–297
Deban L, Jarva H, Lehtinen MJ, Bottazzi B, Bastone A, Doni A, Jokiranta TS, Mantovani A, Meri S (2008) Binding of the long pentraxin PTX3 to factor H: interacting domains and function in the regulation of complement activation. J Immunol 181(12):8433–8440
Okemefuna AI, Nan R, Miller A, Gor J, Perkins SJ (2010) Complement factor H binds at two independent sites to C-reactive protein in acute phase concentrations. J Biol Chem 285(2):1053–1065
Peake P, Shen Y (2010) Factor H binds to the N-terminus of adiponectin and modulates complement activation. Biochem Biophys Res Commun 397(2):361–366
Sjoberg AP, Nystrom S, Hammarstrom P, Blom AM (2008) Native, amyloid fibrils and beta-oligomers of the C-terminal domain of human prion protein display differential activation of complement and bind C1q, factor H and C4b-binding protein directly. Mol Immunol 45(11):3213–3221
Mitchell DA, Kirby L, Paulin SM, Villiers CL, Sim RB (2007) Prion protein activates and fixes complement directly via the classical pathway: implications for the mechanism of scrapie agent propagation in lymphoid tissue. Mol Immunol 44(11):2997–3004
Bradford BM, Mabbott NA (2012) Prion disease and the innate immune system. Viruses 4(12):3389–3419
Acknowledgements
This work was supported by National Natural Science Foundation of China (81772197, 81401670, and 81630062), the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (2018RC330004), National Key R&D Program of China (2018YFC1200305 and 2016YFC1202700), SKLID Development Grant (2015SKLID503 and 2016SKLID603), and the Young Scholar Scientific Research Foundation of China CDC (2016A101).
Author information
Authors and Affiliations
Contributions
CC and YL designed the study and drafted the manuscript; CH and RQZ carried out the Western blot; XFX, YM, and LPG carried out the IHC; JLL and QS carried out the IFA; QS carried out the real-time PCR; KX and JW performed the statistical analysis. XPD conceived of the study, participated in its design and coordination, and helped to draft the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that they have no conflict of interest.
Additional information
Edited by Matthias J. Reddehase.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.

430_2019_641_MOESM1_ESM.jpg
Supplementary Fig. 1. Control experiments. (A) Immunofluorescent staining with 2nd-Ab only in brain slices of age-matched healthy control and 139A-infected mice at terminal stage of prion infection (63×). Various 2nd-Ab are indicated on the top. (B) IHC staining with 2nd-Ab only in brain slices of age-matched healthy control, 139A-infected mice and 263 K-infected hamsters at terminal stage of prion infection (40×). Various brain regions are indicated on the top. Brain slices from 3 mice/hamsters in each group (JPEG 2558 kb)


430_2019_641_MOESM3_ESM.jpg
Supplementary Fig. 2. Hierarchical scanning of the colocalization between CFB (green) and Iba1 (red) in the cortex of control and 139A-infected mice at end stage of prion infection (63×). (A) age-matched healthy control, (B) 139A-infected mice. The scale is shown at the bottom right of each image. Brain slices from 3 mice/hamsters in each group (JPEG 3502 kb)
Rights and permissions
About this article
Cite this article
Chen, C., Lv, Y., Hu, C. et al. Alternative complement pathway is activated in the brains of scrapie-infected rodents. Med Microbiol Immunol 209, 81–94 (2020). https://doi.org/10.1007/s00430-019-00641-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00430-019-00641-6