Abstract
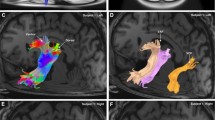
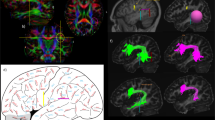
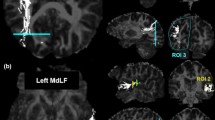
Historically, the primary focus of studies of human white matter tracts has been on large tracts that connect anterior-to-posterior cortical regions. These include the superior longitudinal fasciculus (SLF), the inferior longitudinal fasciculus (ILF), and the inferior fronto-occipital fasciculus (IFOF). Recently, more refined and well-understood tractography methods have facilitated the characterization of several tracts in the posterior of the human brain that connect dorsal-to-ventral cortical regions. These include the vertical occipital fasciculus (VOF), the posterior arcuate fasciculus (pArc), the temporo-parietal connection (TP-SPL), and the middle longitudinal fasciculus (MdLF). The addition of these dorso-ventral connective tracts to our standard picture of white matter architecture results in a more complicated pattern of white matter connectivity than previously considered. Dorso-ventral connective tracts may play a role in transferring information from superior horizontal tracts, such as the SLF, to inferior horizontal tracts, such as the IFOF and ILF. We present a full anatomical delineation of these major dorso-ventral connective white matter tracts (the VOF, pArc, TP-SPL, and MdLF). We show their spatial layout and cortical termination mappings in relation to the more established horizontal tracts (SLF, IFOF, ILF, and Arc) and consider standard values for quantitative features associated with the aforementioned tracts. We hope to facilitate further study on these tracts and their relations. To this end, we also share links to automated code that segments these tracts, thereby providing a standard approach to obtaining these tracts for subsequent analysis. We developed open source software to allow reproducible segmentation of the tracts: https://github.com/brainlife/Vertical_Tracts. Finally, we make the segmentation method available as an open cloud service on the data and analyses sharing platform brainlife.io. Investigators will be able to access these services and upload their data to segment these tracts.
















Similar content being viewed by others
References
Ajina S, Pestilli F, Rokem A et al (2015) Human blindsight is mediated by an intact geniculo-extrastriate pathway. Elife. https://doi.org/10.7554/elife.08935
Allen B, Spiegel DP, Thompson B et al (2015) Altered white matter in early visual pathways of humans with amblyopia. Vis Res 114:48–55
Avesani P, McPherson B, Hayashi S et al (2019) The open diffusion data derivatives, brain data upcycling via integrated publishing of derivatives and reproducible open cloud services. Sci Data 6:69
Axer M, Grässel D, Kleiner M et al (2011) High-Resolution Fiber Tract Reconstruction in the Human Brain by Means of Three-Dimensional Polarized Light Imaging. Front Neuroinform 5:34. https://doi.org/10.3389/fninf.2011.00034
Bajada CJ, Lambon Ralph MA, Cloutman LL (2015) Transport for language south of the Sylvian fissure: the routes and history of the main tracts and stations in the ventral language network. Cortex 69:141–151
Bartolomeo P, Thiebaut de Schotten M, Chica AB (2012) Brain networks of visuospatial attention and their disruption in visual neglect. Front Hum Neurosci 6:110
Bartsch AJ, Geletneky K, Jbabdi S (2013) The temporoparietal fiber intersection area and wernicke perpendicular fasciculus. Neurosurgery 73:E381–E382
Basser PJ, Jones DK (2002) Diffusion-tensor MRI: theory, experimental design and data analysis—a technical review. NMR Biomed 15:456–467
Basser PJ, Pajevic S, Pierpaoli C et al (2000) In vivo fiber tractography using DT-MRI data. Magn Reson Med 44:625–632
Behrens TEJ, Woolrich MW, Jenkinson M et al (2003) Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med 50:1077–1088
Broca P (1865) Sur le siège de la faculté du langage articulé. Bull Mem Soc Anthropol Paris 6:377–393
Budisavljevic S, Dell’Acqua F, Castiello U (2018) Cross-talk connections underlying dorsal and ventral stream integration during hand actions. Cortex. https://doi.org/10.1016/j.cortex.2018.02.016
Bullmore E, Sporns O (2009) Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 10:186–198
Caiafa CF, Pestilli F (2017) Multidimensional encoding of brain connectomes. Sci Rep 7:11491
Catani M, de Schotten MT (2012) Atlas of human brain connections (all tracts). In: Atlas of human brain connections. Oxford University Press, pp 75–238
Catani M, Ffytche DH (2005) The rises and falls of disconnection syndromes. Brain 128:2224–2239
Catani M, Mesulam M (2008) The arcuate fasciculus and the disconnection theme in language and aphasia: history and current state. Cortex 44:953–961
Catani M, Thiebaut de Schotten M (2008) A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex 44:1105–1132
Catani M, Howard RJ, Pajevic S, Jones DK (2002) Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage 17:77–94
Catani M, Jones DK, Ffytche DH (2005) Perisylvian language networks of the human brain. Ann Neurol 57:8–16
Catani M, Bodi I, Dell’Acqua F (2012) Comment on “The geometric structure of the brain fiber pathways”. Science 337:1605
Catani M, Mesulam MM, Jakobsen E et al (2013) A novel frontal pathway underlies verbal fluency in primary progressive aphasia. Brain 136:2619–2628
Charcot JM (2016) Lectures on the localisation of cerebral and spinal diseases. Palala Press, Newark (Tr. and Ed. by W.B. Hadden)
Curran EJ (1909) A new association fiber tract in the cerebrum with remarks on the fiber tract dissection method of studying the brain. J Comp Neurol Psychol 19:645–656
Davis LE (1921) An anatomic study of the inferior longitudinal fasciculus. Arch Neurol Psychiatry 5:370
De Benedictis A, Duffau H, Paradiso B et al (2014) Anatomo-functional study of the temporo-parieto-occipital region: dissection, tractographic and brain mapping evidence from a neurosurgical perspective. J Anat 225:132–151
De Benedictis A, Petit L, Descoteaux M et al (2016) New insights in the homotopic and heterotopic connectivity of the frontal portion of the human corpus callosum revealed by microdissection and diffusion tractography. Hum Brain Mapp 37:4718–4735
de Schotten MT, Dell’Acqua F, Forkel SJ et al (2011) A lateralized brain network for visuospatial attention. Nat Neurosci 14:1245–1246
Decramer T, Swinnen S, van Loon J et al (2018) White matter tract anatomy in the rhesus monkey: a fiber dissection study. Brain Struct Funct 223:3681–3688
Dejerine J, Dejerine-Klumpke A (1895) Anatomie des centres nerveux. Rueff & Company, Paris
Descoteaux M, Deriche R, Knösche TR, Anwander A (2009) Deterministic and probabilistic tractography based on complex fibre orientation distributions. IEEE Trans Med Imaging 28:269–286
Destrieux C, Fischl B, Dale A, Halgren E (2010) Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage 53:1–15
Dick AS, Tremblay P (2012) Beyond the arcuate fasciculus: consensus and controversy in the connectional anatomy of language. Brain 135:3529–3550
Dick AS, Bernal B, Tremblay P (2014) The language connectome: new pathways, new concepts. Neuroscientist 20:453–467
Dohmen M, Menzel M, Wiese H et al (2015) Understanding fiber mixture by simulation in 3D polarized light imaging. Neuroimage 111:464–475
Edinger L (1885) Über den Verlauf der centralen Hirnnervenbahnen mit Demonstrationen von Präparaten. Arch Psychiatr Nervenkr 16:858–859
Edinger L (1893) The significance of the cortex considered in connection with a report upon a dog from which the entire cerebrum had been removed by Prof. Goltz. J Comp Neurol 3:69–77
Fang Y, Wang X, Zhong S et al (2018) Semantic representation in the white matter pathway. PLoS Biol 16:e2003993
Fernández-Miranda JC, Rhoton AL Jr, Alvarez-Linera J et al (2008) Three-dimensional microsurgical and tractographic anatomy of the white matter of the human brain. Neurosurgery 62:989–1026 (discussion 1026–8)
Fields RD (2008a) White matter in learning, cognition and psychiatric disorders. Trends Neurosci 31:361–370
Fields RD (2008b) White matter matters. Sci Am 298:54–61
Fischl B (2012) FreeSurfer. Neuroimage 62:774–781
Flourens P (1846) Phrenology examined. Am J Med Sci 11:437
Forkel SJ, Thiebaut de Schotten M, Kawadler JM et al (2014) The anatomy of fronto-occipital connections from early blunt dissections to contemporary tractography. Cortex 56:73–84
Friederici AD (2011) The brain basis of language processing: from structure to function. Physiol Rev 91:1357–1392
Gabrieli JDE (2009) Dyslexia: a new synergy between education and cognitive neuroscience. Science 325:280–283
Gall FJ (1810) Anatomie et physiologie du système nerveux en général, et du cerveau en particulier: avec des observations sur la possibilité de reconnaître plusieurs dispositions intellectuelles et morales de l’homme et des animaux, par la configuration de leurs têtes
Garyfallidis E, Brett M, Amirbekian B et al (2014) Dipy, a library for the analysis of diffusion MRI data. Front Neuroinform 8:8
Geschwind N (1965) Disconnexion syndromes in animals and man. I. Brain 88:237–294
Geschwind N (1974) Disconnexion Syndromes in Animals and Man. In: Selected Papers on Language and the Brain. Boston Studies in the Philosophy of Science, vol 16. Springer, Dordrecht, pp 105–236
Glasser MF, Sotiropoulos SN, Wilson JA et al (2013) The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage 80:105–124
Goldstone RL, Pestilli F, Börner K (2015) Self-portraits of the brain: cognitive science, data visualization, and communicating brain structure and function. Trends Cogn Sci 19:462–474
Gomez J, Pestilli F, Witthoft N et al (2015) Functionally defined white matter reveals segregated pathways in human ventral temporal cortex associated with category-specific processing. Neuron 85:216–227
Goodale MA, Milner AD (1992) Separate visual pathways for perception and action. Trends Neurosci 15:20–25
Goryainov SA, Kondrashov AV, Gol’dberg MF et al (2017) Long association tracts of the human white matter: an analysis of 18 hemisphere dissections and in vivo HARDI-CSD tractography. Zh Vopr Neirokhir Im N N Burdenko 81:13–25
Gray H (1918) Anatomy of the human body. Lea & Febiger, Philadelphia
Grill-Spector K, Kushnir T, Edelman S et al (1998) Cue-invariant activation in object-related areas of the human occipital lobe. Neuron 21:191–202
Hagmann P, Jonasson L, Maeder P et al (2006) Understanding diffusion mr imaging techniques: from scalar diffusion-weighted imaging to diffusion tensor imaging and beyond. Radiographics 26:S205–S223
Hagmann P, Cammoun L, Gigandet X et al (2008) Mapping the structural core of human cerebral cortex. PLoS Biol 6:e159
Hart J (2015) White matter and cognition. In: The Neurobiology of Cognition and Behavior. Oxford University Press, pp 169–186
Hau J, Sarubbo S, Houde JC et al (2017) Revisiting the human uncinate fasciculus, its subcomponents and asymmetries with stem-based tractography and microdissection validation. Brain Struct Funct 222:1645–1662
Haxby JV, Hoffman EA, Ida Gobbini M (2000) The distributed human neural system for face perception. Trends Cogn Sci 4:223–233
Homola GA, Jbabdi S, Beckmann CF, Bartsch AJ (2012) A brain network processing the age of faces. PLoS One 7:e49451
Honey CJ, Sporns O (2008) Dynamical consequences of lesions in cortical networks. Hum Brain Mapp 29:802–809
Hubel DH, Livingstone MS (1987) Segregation of form, color, and stereopsis in primate area 18. J Neurosci 7:3378–3415
Jbabdi S, Johansen-Berg H (2011) Tractography: where do we go from here? Brain Connect 1:169–183
Jbabdi S, Sotiropoulos SN, Haber SN et al (2015) Measuring macroscopic brain connections in vivo. Nat Neurosci 18:1546–1555
Kamali A, Flanders AE, Brody J et al (2014a) Tracing superior longitudinal fasciculus connectivity in the human brain using high resolution diffusion tensor tractography. Brain Struct Funct 219:269–281
Kamali A, Sair HI, Radmanesh A, Hasan KM (2014b) Decoding the superior parietal lobule connections of the superior longitudinal fasciculus/arcuate fasciculus in the human brain. Neuroscience 277:577–583
Kanwisher N (2010) Functional specificity in the human brain: a window into the functional architecture of the mind. Proc Natl Acad Sci 107:11163–11170
Lawes INC, Barrick TR, Murugam V et al (2008) Atlas-based segmentation of white matter tracts of the human brain using diffusion tensor tractography and comparison with classical dissection. Neuroimage 39:62–79
Lazar M, Weinstein DM, Tsuruda JS et al (2003) White matter tractography using diffusion tensor deflection. Hum Brain Mapp 18:306–321
Lee Masson H, Wallraven C, Petit L (2017) “Can touch this”: cross-modal shape categorization performance is associated with microstructural characteristics of white matter association pathways. Hum Brain Mapp 38:842–854
Leong JK, Pestilli F, Wu CC et al (2016) White-matter tract connecting anterior insula to nucleus accumbens correlates with reduced preference for positively skewed gambles. Neuron 89:63–69
Libero LE, Burge WK, Deshpande HD et al (2016) White matter diffusion of major fiber tracts implicated in autism spectrum disorder. Brain Connect 6:691–699
Lunven M, Thiebaut De Schotten M, Bourlon C et al (2015) White matter lesional predictors of chronic visual neglect: a longitudinal study. Brain 138:746–760
Mai JK, Majtanik M, Paxinos G (2015) Atlas of the human brain. Academic Press, London
Maier-Hein KH, Neher PF, Houde J-C et al (2017) The challenge of mapping the human connectome based on diffusion tractography. Nat Commun 8:1349
Makris N, Kennedy DN, McInerney S et al (2004) Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb Cortex 15:854–869
Makris N, Papadimitriou GM, Kaiser JR et al (2009) Delineation of the middle longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb Cortex 19:777–785
Makris N, Preti MG, Asami T et al (2013a) Human middle longitudinal fascicle: variations in patterns of anatomical connections. Brain Struct Funct 218:951–968
Makris N, Preti MG, Wassermann D et al (2013b) Human middle longitudinal fascicle: segregation and behavioral-clinical implications of two distinct fiber connections linking temporal pole and superior temporal gyrus with the angular gyrus or superior parietal lobule using multi-tensor tractography. Brain Imaging Behav 7:335–352
Makris N, Zhu A, Papadimitriou GM et al (2017) Mapping temporo-parietal and temporo-occipital cortico-cortical connections of the human middle longitudinal fascicle in subject-specific, probabilistic, and stereotaxic Talairach spaces. Brain Imaging Behav 11:1258–1277
Maldonado IL, de Champfleur NM, Velut S et al (2013) Evidence of a middle longitudinal fasciculus in the human brain from fiber dissection. J Anat 223:38–45
Martino J, De Lucas EM (2014) Subcortical anatomy of the lateral association fascicles of the brain: a review. Clin Anat 27:563–569
Martino J, García-Porrero JA (2013) Wernicke perpendicular fasciculus and vertical portion of the superior longitudinal fasciculus. Neurosurgery 73:E382–E383
Martino J, Brogna C, Robles SG et al (2010a) Anatomic dissection of the inferior fronto-occipital fasciculus revisited in the lights of brain stimulation data. Cortex 46:691–699
Martino J, Vergani F, Robles SG, Duffau H (2010b) New insights into the anatomic dissection of the temporal stem with special emphasis on the inferior fronto-occipital fasciculus: implications in surgical approach to left mesiotemporal and temporoinsular structures. Neurosurgery 66:4–12
Martino J, da Silva-Freitas R, Caballero H et al (2013a) Fiber dissection and diffusion tensor imaging tractography study of the temporoparietal fiber intersection area. Neurosurgery 72:87–97 (discussion 97–8)
Martino J, De Witt Hamer PC, Berger MS et al (2013b) Analysis of the subcomponents and cortical terminations of the perisylvian superior longitudinal fasciculus: a fiber dissection and DTI tractography study. Brain Struct Funct 218:105–121
Menjot de Champfleur N, Lima Maldonado I, Moritz-Gasser S et al (2013) Middle longitudinal fasciculus delineation within language pathways: a diffusion tensor imaging study in human. Eur J Radiol 82:151–157
Miller BL (2010) A commentary on “Disconnexion syndromes in animals and man”. Neuropsychol Rev 20:126–127
Milner AD, Goodale MA (2008) Two visual systems re-viewed. Neuropsychologia 46:774–785
Mori S, Zhang J (2006) Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron 51:527–539
Mori S, Crain BJ, Chacko VP, van Zijl PC (1999) Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol 45:265–269
Mori S, Kaufmann WE, Davatzikos C et al (2002) Imaging cortical association tracts in the human brain using diffusion-tensor-based axonal tracking. Magn Reson Med 47:215–223
Mori S, Wakana S, van Zijl PCM, Nagae-Poetscher LM (2005) MRI atlas of human white matter. Elsevier, Amsterdam
Obersteiner H (1889) Anleitung beim Studium des Baues der nervosen Centralorgane im gesunden und kranken Zustande. Am J Psychol 2:304
Obersteiner H (1890) The anatomy of the central nervous organs in health and disease. Charles Griffin & Company, London
Ogawa S, Takemura H, Horiguchi H et al (2014) White matter consequences of retinal receptor and ganglion cell damage. Investig Ophthalmol Vis Sci 55:6976–6986
Panesar SS, Belo JTA, Yeh F-C, Fernandez-Miranda JC (2019) Structure, asymmetry, and connectivity of the human temporo-parietal aslant and vertical occipital fasciculi. Brain Struct Funct 224:907–923
Pestilli F (2018) Human white matter and knowledge representation. PLoS Biol 16:e2005758
Pestilli F, Bullock D (2019) Associative white matter connecting the dorsal and ventral posterior human cortex. brainlife.io. https://doi.org/10.25663/brainlife.pub.4
Pestilli F, Yeatman JD, Rokem A et al (2014) Evaluation and statistical inference for human connectomes. Nat Methods 11:1058–1063
Petrides M, Pandya DN (2009) Distinct parietal and temporal pathways to the homologues of Broca’s area in the monkey. PLoS Biol 7:e1000170
Ptak R, Schnider A (2010) The dorsal attention network mediates orienting toward behaviorally relevant stimuli in spatial neglect. J Neurosci 30:12557–12565
Reese TG, Heid O, Weisskoff RM, Wedeen VJ (2003) Reduction of eddy-current-induced distortion in diffusion MRI using a twice-refocused spin echo. Magn Reson Med 49:177–182
Reveley C, Seth AK, Pierpaoli C et al (2015) Superficial white matter fiber systems impede detection of long-range cortical connections in diffusion MR tractography. Proc Natl Acad Sci USA 112:E2820–E2828
Rokem A, Yeatman JD, Pestilli F et al (2015) Evaluating the accuracy of diffusion MRI models in white matter. PLoS One 10:e0123272
Rokem A, Takemura H, Bock AS et al (2017) The visual white matter: the application of diffusion MRI and fiber tractography to vision science. J Vis 17:4
Rubner Y, Tomasi C, Guibas LJ (2000) The earth mover’s distance as a metric for image retrieval. Int J Comput Vis 40:99–121
Saber GT, Pestilli F, Curtis CE (2015) Saccade planning evokes topographically specific activity in the dorsal and ventral streams. J Neurosci 35:245–252
Sakata H, Taira M, Kusunoki M et al (1997) The TINS lecture. The parietal association cortex in depth perception and visual control of hand action. Trends Neurosci 20:350–357
Sani I, McPherson BC, Stemmann H et al (2019) Functionally defined white matter of the macaque monkey brain reveals a dorso-ventral attention network. Elife. https://doi.org/10.7554/elife.40520
Sarubbo S, De Benedictis A, Maldonado IL et al (2013) Frontal terminations for the inferior fronto-occipital fascicle: anatomical dissection, DTI study and functional considerations on a multi-component bundle. Brain Struct Funct 218:21–37
Sarubbo S, De Benedictis A, Merler S et al (2016) Structural and functional integration between dorsal and ventral language streams as revealed by blunt dissection and direct electrical stimulation. Hum Brain Mapp 37:3858–3872
Schmahmann JD, Pandya DN (2006) Fiber pathways of the brain. Oxford University Press
Schmahmann JD, Pandya DN (2007) Cerebral white matter—historical evolution of facts and notions concerning the organization of the fiber pathways of the brain. J Hist Neurosci 16:237–267
Schmahmann JD, Pandya DN, Wang R et al (2007) Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain 130:630–653
Scholz J, Klein M, Behrens T, Johansen-Berg H (2009) White matter microstructure changes in response to training. NeuroImage 47:S77
Sotiropoulos SN, Jbabdi S, Xu J et al (2013) Advances in diffusion MRI acquisition and processing in the Human Connectome Project. Neuroimage 80:125–143
Steinmetz NA, Moore T (2014) Eye movement preparation modulates neuronal responses in area V4 when dissociated from attentional demands. Neuron 83:496–506
Stricker S (1871) Handbuch der Lehre von den Geweben des Menschen und der Thiere. Wilhelm Engelmann, Leipzig
Takemura H, Caiafa CF, Wandell BA, Pestilli F (2016a) Ensemble tractography. PLoS Comput Biol 12:e1004692
Takemura H, Rokem A, Winawer J et al (2016b) A major human white matter pathway between dorsal and ventral visual cortex. Cereb Cortex 26:2205–2214
Takemura H, Pestilli F, Weiner KS et al (2017) Occipital white matter tracts in human and macaque. Cereb Cortex. https://doi.org/10.1093/cercor/bhx070
Takemura H, Pestilli F, Weiner KS (2018) Comparative neuroanatomy: integrating classic and modern methods to understand association fibers connecting dorsal and ventral visual cortex. Neurosci Res. https://doi.org/10.1016/j.neures.2018.10.011
Thomas C, Ye FQ, Irfanoglu MO et al (2014) Anatomical accuracy of brain connections derived from diffusion MRI tractography is inherently limited. Proc Natl Acad Sci USA 111:16574–16579
Thomason ME, Thompson PM (2011) Diffusion imaging, white matter, and psychopathology. Annu Rev Clin Psychol 7:63–85
Thompson PM, Stein JL, Medland SE et al (2014) The ENIGMA Consortium: large-scale collaborative analyses of neuroimaging and genetic data. Brain Imaging Behav 8:153–182
Tournier J-D, Calamante F, Connelly A (2007) Robust determination of the fibre orientation distribution in diffusion MRI: non-negativity constrained super-resolved spherical deconvolution. Neuroimage 35:1459–1472
Tournier J-D, Calamante F, Connelly A (2012) MRtrix: diffusion tractography in crossing fiber regions. Int J Imaging Syst Technol 22:53–66
Turken AU, Dronkers NF (2011) The neural architecture of the language comprehension network: converging evidence from lesion and connectivity analyses. Front Syst Neurosci 5:1
Uesaki M, Takemura H, Ashida H (2018) Computational neuroanatomy of human stratum proprium of interparietal sulcus. Brain Struct Funct 223:489–507
Ungerleider L, Haxby J (1994) “What” and “where” in the human brain. Curr Opin Neurobiol 4:157–165
Van Essen DC, Ugurbil K, Auerbach E et al (2012) The human connectome project: a data acquisition perspective. Neuroimage 62:2222–2231
Ventura-Antunes L, Mota B, Herculano-Houzel S (2013) Different scaling of white matter volume, cortical connectivity, and gyrification across rodent and primate brains. Front Neuroanat 7:3
Wakana S, Caprihan A, Panzenboeck MM et al (2007) Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage 36:630–644
Wandell BA (2016) Clarifying human white matter. Annu Rev Neurosci 39:103–128
Wang Y, Fernández-Miranda JC, Verstynen T et al (2013) Rethinking the role of the middle longitudinal fascicle in language and auditory pathways. Cereb Cortex 23:2347–2356
Wang X, Pathak S, Stefaneanu L et al (2016) Subcomponents and connectivity of the superior longitudinal fasciculus in the human brain. Brain Struct Funct 221:2075–2092
Wassermann D, Makris N, Rathi Y et al (2013) On describing human white matter anatomy: the white matter query language. Med Image Comput Comput Assist Interv 16:647–654
Wassermann D, Makris N, Rathi Y et al (2016) The white matter query language: a novel approach for describing human white matter anatomy. Brain Struct Funct 221:4705–4721
Wasserthal J, Neher P, Maier-Hein KH (2018) TractSeg—fast and accurate white matter tract segmentation. Neuroimage 183:239–253
Wedeen VJ, Rosene DL, Wang R et al (2012) The geometric structure of the brain fiber pathways. Science 335:1628–1634
Weiner KS, Yeatman JD, Wandell BA (2016) The posterior arcuate fasciculus and the vertical occipital fasciculus. Cortex. https://doi.org/10.1016/j.cortex.2016.03.012
Wernicke C (1874) Der aphasische Symptomencomplex: eine psychologische Studie auf anatomischer Basis. Max Cohn & Weigert, Breslau
Wernicke C (1881) Lehrbuch der Gehirnkrankheiten für Aerzte und Studirende. Theodor Fischer, Kassel
Wu Y, Sun D, Wang Y et al (2016) Tracing short connections of the temporo-parieto-occipital region in the human brain using diffusion spectrum imaging and fiber dissection. Brain Res 1646:152–159
Yeatman JD, Dougherty RF, Rykhlevskaia E et al (2011) Anatomical properties of the arcuate fasciculus predict phonological and reading skills in children. J Cogn Neurosci 23:3304–3317
Yeatman JD, Dougherty RF, Myall NJ et al (2012) Tract profiles of white matter properties: automating fiber-tract quantification. PLoS One 7:e49790
Yeatman JD, Rauschecker AM, Wandell BA (2013) Anatomy of the visual word form area: adjacent cortical circuits and long-range white matter connections. Brain Lang 125:146–155
Yeatman JD, Weiner KS, Pestilli F et al (2014) The vertical occipital fasciculus: a century of controversy resolved by in vivo measurements. Proc Natl Acad Sci USA 111:E5214–E5223
Yeatman JD, Richie-Halford A, Smith JK et al (2018) A browser-based tool for visualization and analysis of diffusion MRI data. Nat Commun 9:940
Yeh F-C, Panesar S, Fernandes D et al (2018) Population-averaged atlas of the macroscale human structural connectome and its network topology. NeuroImage 178:57–68
Zhang K, Sejnowski TJ (2000) A universal scaling law between gray matter and white matter of cerebral cortex. Proc Natl Acad Sci USA 97:5621–5626
Zola-Morgan S (1995) Localization of brain function: the legacy of Franz Joseph Gall (1758–1828). Annu Rev Neurosci 18:359–383
Acknowledgements
This research was supported by NSF IIS-1636893, NSF BCS-1734853, NSF AOC 1916518, NIH NCATS UL1TR002529, a Microsoft Research Award, Google Cloud Platform, Japan Society for the Promotion of Science (JSPS) KAKENHI (JP17H04684, JP15J00412), and the Indiana University Areas of Emergent Research initiative “Learning: Brains, Machines, Children.” In part by NIH NCATS UL1TR002529 to F.P.. D.N.B. and B.M. were partially funded via NIH NIMH 5 T32 MH103213 to B. Hetrick and B. D’Onofrio. We thank Soichi Hayashi, Steven O’Riley, David Hunt, and Aman Arya for contributing to the development of brainlife.io, Craig Stewart, Winona Snapp-Childs, David Hancok, and Jeremy Fischer for support with jetstream-cloud.org (NSF ACI-1445604). Data were provided in part by the Human Connectome Project, WU-Minn Consortium (Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657) funded by the 16 NIH Institutes and Centers that support the NIH Blueprint for Neuroscience Research; and by the McDonnell Center for Systems Neuroscience at Washington University. Data were provided in part by Brian Wandell (Stanford University; https://purl.stanford.edu/bb060nk0241). Thanks to Sophia Vinci-Booher for comments on early versions of the manuscript. Thanks also to Josh Faskowitz for help with software.
Author information
Authors and Affiliations
Contributions
DNB and FP conceptualized and performed analyses. HT and CC provided data curation and software. DNB, HT, CC, and FP wrote the manuscript. LK, BM, and BC provided validation and software.
Corresponding author
Ethics declarations
Conflicts of interest
Authors declare no conflicts of interest.
Research involving human participants and/or animals
Data collection was approved by the respective Institutional Review Boards (IRBs) of the University of Washington, Saint Louis (HCP data set) and Stanford University (STN data set).
Informed consent
All participants provided written informed consent to participate in the project.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bullock, D., Takemura, H., Caiafa, C.F. et al. Associative white matter connecting the dorsal and ventral posterior human cortex. Brain Struct Funct 224, 2631–2660 (2019). https://doi.org/10.1007/s00429-019-01907-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-019-01907-8