Abstract
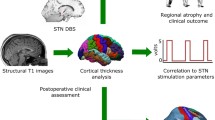
We investigated whether pre-operative MRI measures of focal brain atrophy could predict cognitive decline occurring after deep brain stimulation (DBS) of the subthalamic nucleus (STN) in patients with Parkinson’s disease (PD). For that purpose, we prospectively collected data of 42 consecutive patients with PD who underwent bilateral STN-DBS. Normalized brain structure volumes and cortical thicknesses were measured on pre-operative T1-weighted MRI. Patients were tested for their cognitive performances before surgery and 1 year after. After controlling for age, gender, pre-operative disease severity, change in dopaminomimetic dose after surgery and contact location, we found correlations: (1) between the variation of the total Mattis dementia rating scale (MDRS) score and left lateral ventricle volume (p = 0.032), (2) between the variation of the initiation/perseveration subscore of the MDRS and the left nucleus accumbens volume (p = 0.042) and the left lateral ventricle volume (p = 0.017) and (3) between the variation of the backward digit-span task and the right and left superior frontal gyrus thickness (p = 0.004 and p = 0.007, respectively). Left nucleus accumbens atrophy was associated with decline in the initiation/perseveration subscore with the largest effect size (d = − 1.64). Pre-operative left nucleus accumbens volume strongly predicted postoperative decline in the initiation/attention subscore (AUC = 0.92, p < 0.001, 96.3% sensitivity, 80.0% specificity, 92.9% PPV and 92.9% NPV). We conclude that the morphometric measures of brain atrophy usually associated with cognitive impairment in PD can also explain or predict a part of cognitive decline after bilateral STN-DBS. In particular, the left accumbens nucleus volume could be considered as a promising marker for guiding surgical decisions.



Similar content being viewed by others
References
Alegret M, Junqué C, Valldeoriola F et al (2001) Effects of bilateral subthalamic stimulation on cognitive function in Parkinson disease. Arch Neurol 58:1223–1227
Altman DG (1990) Practical statistics for medical research. CRC Press, Boca Raton
Aybek S, Lazeyras F, Gronchi-Perrin A et al (2009) Hippocampal atrophy predicts conversion to dementia after STN-DBS in Parkinson’s disease. Parkinsonism Relat Disord 15:521–524
Bonneville F, Welter ML, Elie C et al (2005) Parkinson disease, brain volumes, and subthalamic nucleus stimulation. Neurology 64:1598–1604
Camicioli R, Sabino J, Gee M et al (2011) Ventricular dilatation and brain atrophy in patients with Parkinson’s disease with incipient dementia. Mov Disord 26:1443–1450
Carriere N, Besson P, Dujardin K et al (2014) Apathy in Parkinson’s disease is associated with nucleus accumbens atrophy: a magnetic resonance imaging shape analysis. Mov Disord 29:897–903
Charles PD, Van Blercom N, Krack P et al (2002) Predictors of effective bilateral subthalamic nucleus stimulation for PD. Neurology 59:932–934
Contarino MF, Daniele A, Sibilia AH et al (2007) Cognitive outcome 5 years after bilateral chronic stimulation of subthalamic nucleus in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry 78:248–252
Daniels C, Krack P, Volkmann J et al (2010) Risk factors for executive dysfunction after subthalamic nucleus stimulation in Parkinson’s disease. Mov Disord 25:1583–1589
de Chazeron I, Pereira B, Chereau-Boudet I et al (2016) Impact of localisation of deep brain stimulation electrodes on motor and neurobehavioural outcomes in Parkinson’s disease. J Neurol Neurosurg Psychiatry 87:758–766
Desikan RS, Ségonne F, Fischl B et al (2006) An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 31:968–980
Dujardin K, Devos D, Duhem S et al (2006) Utility of the Mattis dementia rating scale to assess the efficacy of rivastigmine in dementia associated with Parkinson’s disease. J Neurol 253:1154–1159
Emre M (2004) Dementia in Parkinson’s disease: cause and treatment. Curr Opin Neurol 17:399–404
Fischl B, Dale AM (2000) Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA 97:11050–11055
Funkiewiez A, Ardouin C, Caputo E et al (2004) Long term effects of bilateral subthalamic nucleus stimulation on cognitive function, mood, and behaviour in Parkinson’s disease. J Neurol Neurosurg Psychiatry 75:834–839
Hanganu A, Bedetti C, Degroot C et al (2014) Mild cognitive impairment is linked with faster rate of cortical thinning in patients with Parkinson’s disease longitudinally. Brain 137:1120–1129
Houvenaghel J-F, Le Jeune F, Dondaine T et al (2015) Reduced verbal fluency following subthalamic deep brain stimulation: a frontal-related cognitive deficit? PloS One 10:e0140083
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55:181–184
Ibarretxe-Bilbao N, Junque C, Segura B et al (2012) Progression of cortical thinning in early Parkinson’s disease. Mov Disord 27:1746–1753
Kim H-J, Jeon BS, Paek SH et al (2014) Long-term cognitive outcome of bilateral subthalamic deep brain stimulation in Parkinson’s disease. J Neurol 261:1090–1096
Krack P, Batir A, Van Blercom N et al (2003) Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N Engl J Med 349:1925–1934
Lang AE, Houeto J-L, Krack P et al (2006) Deep brain stimulation: preoperative issues. Mov Disord 21(Suppl 14):S171–S196
Le Goff F, Derrey S, Lefaucheur R et al (2015) Decline in verbal fluency after subthalamic nucleus deep brain stimulation in Parkinson’s disease: a microlesion effect of the electrode trajectory? J Park Dis 5:95–104
Lemaire J-J, Coste J, Ouchchane L et al (2007) Brain mapping in stereotactic surgery: a brief overview from the probabilistic targeting to the patient-based anatomic mapping. NeuroImage 37(Suppl 1):S109-115
Lemaire J-J, Pereira B, Derost P et al (2016) Subthalamus stimulation in Parkinson disease: accounting for the bilaterality of contacts. Surg Neurol Int 7:S837–S847
Mak E, Su L, Williams GB et al (2015) Baseline and longitudinal grey matter changes in newly diagnosed Parkinson’s disease: ICICLE-PD study. Brain 138:2974–2986
Manjón JV, Coupé P (2016) volBrain: an online MRI brain volumetry system. Front Neuroinform 10:30
Markser A, Maier F, Lewis CJ et al (2015) Deep brain stimulation and cognitive decline in Parkinson’s disease: the predictive value of electroencephalography. J Neurol 262:2275–2284. d
Marson DC, Dymek MP, Duke LW, Harrell LE (1997) Subscale validity of the Mattis dementia rating scale. Arch Clin Neuropsychol 12:269–275
Næss-Schmidt E, Tietze A, Blicher JU et al (2016) Automatic thalamus and hippocampus segmentation from MP2RAGE: comparison of publicly available methods and implications for DTI quantification. Int J Comput Assist Radiol Surg 11:1979–1991
Parsons TD, Rogers SA, Braaten AJ et al (2006) Cognitive sequelae of subthalamic nucleus deep brain stimulation in Parkinson’s disease: a meta-analysis. Lancet Neurol 5:578–588
Pessoa L (2009) How do emotion and motivation direct executive control? Trends Cognit Sci 13:160–166
Pigott K, Rick J, Xie SX et al (2015) Longitudinal study of normal cognition in Parkinson disease. Neurology 85:1276–1282
Saint-Cyr JA, Trépanier LL, Kumar R et al (2000) Neuropsychological consequences of chronic bilateral stimulation of the subthalamic nucleus in Parkinson’s disease. Brain 123(Pt 10):2091–2108
Schuepbach WMM, Rau J, Knudsen K et al (2013) Neurostimulation for Parkinson’s disease with early motor complications. N Engl J Med 368:610–622
Segura B, Baggio HC, Marti MJ et al (2014) Cortical thinning associated with mild cognitive impairment in Parkinson’s disease. Mov Disord 29:1495–1503
Smeding HMM, Speelman JD, Huizenga HM et al (2011) Predictors of cognitive and psychosocial outcome after STN DBS in Parkinson’s Disease. J Neurol Neurosurg Psychiatry 82:754–760
Thobois S (2006) Proposed dose equivalence for rapid switch between dopamine receptor agonists in Parkinson’s disease: a review of the literature. Clin Ther 28:1–12
Tsai S-T, Lin S-H, Lin S-Z et al (2007) Neuropsychological effects after chronic subthalamic stimulation and the topography of the nucleus in Parkinson’s disease. Neurosurgery 61:E1024–E1029 (discussion E1029–E1030).
Welter ML, Houeto JL, Tezenas du Montcel S et al (2002) Clinical predictive factors of subthalamic stimulation in Parkinson’s disease. Brain 125:575–583
Welter M-L, Schüpbach M, Czernecki V et al (2014) Optimal target localization for subthalamic stimulation in patients with Parkinson disease. Neurology 82:1352–1361
Witt K, Daniels C, Reiff J et al (2008) Neuropsychological and psychiatric changes after deep brain stimulation for Parkinson’s disease: a randomised, multicentre study. Lancet Neurol 7:605–614
Witt K, Granert O, Daniels C et al (2013) Relation of lead trajectory and electrode position to neuropsychological outcomes of subthalamic neurostimulation in Parkinson’s disease: results from a randomized trial. Brain 136:2109–2119
Acknowledgements
The authors want to thank Christine Delaigue, coordinating nurse for the Parkinson’s Disease Center in Clermont-Ferrand University Hospital. They also thank Prof. Thomas Tourdias and Prof. Vincent Dousset from Bordeaux University Hospital for making available their MRI analysis facilities, and Celine Lambert from Clermont-Ferrand University Hospital for her technical assistance.
Funding
Hospital Program for Clinical Research at Clermont-Ferrand University Hospital.
Author information
Authors and Affiliations
Contributions
Conception of the project: VP and FD. Organization: FD. Execution: all authors. Statistical Analysis: VP and BP. Writing of the first draft: VP. Review and Critique: all authors. Study concept and design: VP, BP, JJL and FD.
Corresponding author
Ethics declarations
Conflict of interest
VP received travel expenses and/or consulting fees from the ARSEP Foundation, Biogen, Teva-Lundbeck and Merk-Serono, unrelated to the submitted work. FD serves on scientific advisory boards and has received honoraria and research support for his institution from Novartis, Teva-Lundbeck, Allergan, Aguettant, Servier and Merz, unrelated to the submitted work. Other authors: no conflict of interests.
Rights and permissions
About this article
Cite this article
Planche, V., Munsch, F., Pereira, B. et al. Anatomical predictors of cognitive decline after subthalamic stimulation in Parkinson’s disease. Brain Struct Funct 223, 3063–3072 (2018). https://doi.org/10.1007/s00429-018-1677-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-018-1677-2