Abstract
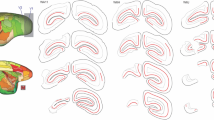
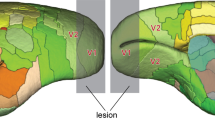
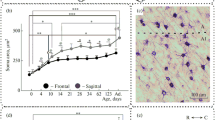
Neuronal loss in the lateral geniculate nucleus (LGN) is a consequence of lesions of the primary visual cortex (V1). Despite the importance of this phenomenon in understanding the residual capacities of the primate visual system following V1 damage, few quantitative studies are available, and the effect of age at the time of lesion remains unknown. We compared the volume, neuronal number, and neuronal density in the LGN, 6–21 months after unilateral V1 lesions in marmoset monkeys. Stereological sampling techniques and neuronal nuclei (NeuN) staining were used to assess the effects of similar-sized lesions in adult (2–4 years) and geriatric (10–14 years) animals. We found that lesions involving the opercular and caudal calcarine parts of V1 caused robust loss of neurons in topographically corresponding regions of the ipsilateral LGN (lesion projection zones), concomitant with a substantial reduction in the volume of this nucleus. Neuronal density was markedly reduced in the lesion projection zones, relative to the corresponding regions of the contralateral LGN, or the LGN in non-lesioned animals. Moreover, the percentage decrease in neuronal density within the lesion projection zones was significantly greater in the geriatric group, compared with the adult groups. The volume and neuronal density in the contralateral LGN of lesioned adult and geriatric marmosets were similar to those in non-lesioned animals. These results show that the primate LGN becomes more vulnerable to degeneration with advancing age. However, even in geriatric primates there is a population of LGN neurons which survives degeneration, and which could play a role in blindsight.






Similar content being viewed by others
References
Ahmad A, Spear PD (1993) Effects of aging on the size, density, and number of rhesus monkey lateral geniculate neurons. J Comp Neurol 334:631–643
Azzopardi P, Cowey A (2001) Motion discrimination in cortically blind patients. Brain 124:30–46
Benevento LA, Yoshida K (1981) The afferent and efferent organization of the lateral geniculo-prestriate pathways in the macaque monkey. J Comp Neurol 203:455–474
Bogousslavsky J, Regli F, van Melle G (1983) Unilateral occipital infarction: evaluation of the risks of developing bilateral loss of vision. J Neurol Neurosurg Psychiatry 46:78–80
Bradley PB (1975) Methods in brain research. Wiley & Sons, New York
Briggs F, Usrey WM (2011) Corticogeniculate feedback and visual processing in the primate. J Physiol 589:33–40. doi:10.1113/jphysiol.2010.193599
Caleo M, Menna E, Chierzi S, Cenni MC, Maffei L (2000) Brain-derived neurotrophic factor is an anterograde survival factor in the rat visual system. Curr Biol 10:1155–1161
Chaplin TA, Yu HH, Rosa MGP (2013) Representation of the visual field in the primary visual area of the marmoset monkey: magnification factors, point-image size, and proportionality to retinal ganglion cell density. J Comp Neurol 521:1001–1019. doi:10.1002/cne.23215
Cowey A (1964) Projection of the retina on to striate and prestriate cortex in the squirrel monkey, Saimiri sciureus. J Neurophysiol 27:366–393
Cowey A, Alexander I, Stoerig P (2011) Transneuronal retrograde degeneration of retinal ganglion cells and optic tract in hemianopic monkeys and humans. Brain 134:2149–2157. doi:10.1093/brain/awr125
Diaz F, Villena A, Gonzalez P, Requena V, Rius F, Perez De Vargas I (1999) Stereological age-related changes in neurons of the rat dorsal lateral geniculate nucleus. Anat Rec 255:396–400
Dineen JT, Hendrickson AE (1981) Age correlated differences in the amount of retinal degeneration after striate cortex lesions in monkeys. Invest Ophthalmol Vis Sci 21:749–752
Dorph-Petersen KA, Caric D, Saghafi R, Zhang W, Sampson AR, Lewis DA (2009) Volume and neuron number of the lateral geniculate nucleus in schizophrenia and mood disorders. Acta Neuropathol 117:369–384. doi:10.1007/s00401-008-0410-2
Finlay BL, Charvet CJ, Bastille I, Cheung DT, Muniz JA, Silveira LCL (2014) Scaling the primate lateral geniculate nucleus: niche and neurodevelopment in the regulation of magnocellular and parvocellular cell number and nucleus volume. J Comp Neurol 522:1839–1857. doi:10.1002/cne.23505
Fritsches KA, Rosa MGP (1996) Visuotopic organisation of striate cortex in the marmoset monkey (Callithrix jacchus). J Comp Neurol 372:264–282
Gallyas F (1979) Silver staining of myelin by means of physical development. Neurol Res 1:203–209
Giordano S, Darley-Usmar V, Zhang J (2013) Autophagy as an essential cellular antioxidant pathway in neurodegenerative disease. Redox Biol 2:82–90. doi:10.1016/j.redox.2013.12.013.
Gundersen HJ, Jensen EB (1987) The efficiency of systematic sampling in stereology and its prediction. J Microsc 147:229–263
Hagan MA, Rosa MGP, Lui LL (2017) Neural plasticity following lesions of the primate occipital lobe: the marmoset as an animal model for studies of blindsight. Dev Neurobiol, in press. doi:10.1002/dneu.22426.
Hendrickson A, Dineen JT (1982) Hypertrophy of neurons in dorsal lateral geniculate nucleus following striate cortex lesions in infant monkeys. Neurosci Lett 30:217–222
Hendrickson A, Warner CE, Possin D, Huang J, Kwan WC, Bourne JA (2015) Retrograde transneuronal degeneration in the retina and lateral geniculate nucleus of the V1-lesioned marmoset monkey. Brain Struct Funct 220:351–360. doi:10.1007/s00429-013-0659-7.
Hill CS, Coleman MP, Menon DK (2016) Traumatic axonal injury: mechanisms and translational opportunities. Trends Neurosci 39:311–324. doi:10.1016/j.tins.2016.03.002
Hiona A, Leeuwenburgh C (2004) Effects of age and caloric restriction on brain neuronal cell death/survival. Ann N Y Acad Sci 1019:96–105
Kisvárday ZF, Cowey A, Stoerig P, Somogyi P (1991) Direct and indirect retinal input into degenerated dorsal lateral geniculate nucleus after striate cortical removal in monkey: implications for residual vision. Exp Brain Res 86:271–292
LeBlanc J, de Guise E, Gosselin N, Feyz M (2006) Comparison of functional outcome following acute care in young, middle-aged and elderly patients with traumatic brain injury. Brain Inj 20:779–790
Li M, He HG, Shi W, Li J, Lv B, Wang CH, Miao QW, Wang ZC, Wang NL, Walter M, Sabel BA (2012) Quantification of the human lateral geniculate nucleus in vivo using MR imaging based on morphometry: volume loss with age. Am J Neuroradiol 33:915–921. doi:10.3174/ajnr.A2884
Matthews MR (1964) Further observations on transneuronal degeneration in the lateral geniculate nucleus of the macaque monkey. J Anat 98:225–263
Matthews MR, Cowan WM, Powell TP (1960) Transneuronal cell degeneration in the lateral geniculate nucleus of the macaque monkey. J Anat 94:145–169
Mattson MP, Magnus T (2006) Ageing and neuronal vulnerability. Nat Rev Neurosci 7:278–294
Mihailović LT, Cupić D, Dekleva N (1971) Changes in the numbers of neurons and glial cells in the lateral geniculate nucleus of the monkey during retrograde cell degeneration. J Comp Neurol 142:223–229
Mitchell JF, Leopold DA (2015) The marmoset monkey as a model for visual neuroscience. Neurosci Res 93:20–46. doi:10.1016/j.neures.2015.01.008
Nishijima K, Saitoh R, Tanaka S, Ohsato-Suzuki M, Ohno T, Kitajima S (2012) Life span of common marmoset (Callithrix jacchus) at CLEA Japan breeding colony. Biogerontology 13:439–443. doi:10.1007/s10522-012-9388-1
Palomer E, Martín-Segura A, Baliyan S, Ahmed T, Balschun D, Venero C, Martin MG, Dotti CG (2016) Aging triggers a repressive chromatin state at Bdnf promoters in hippocampal neurons. Cell Rep 16:2889–2900. doi:10.1016/j.celrep.2016.08.028.
Paxinos G, Watson C, Petrides M, Rosa MGP, Tokuno H (2012) The marmoset brain in stereotaxic coordinates. Academic Press, New York
Perlson E, Maday S, Fu MM, Moughamian AJ, Holzbaur EL (2010) Retrograde axonal transport: pathways to cell death? Trends Neurosci 33:335–344. doi:10.1016/j.tins.2010.03.006
Polyak S (1933) A contribution to the cerebral representation of the retina. J Comp Neurol 57:541–617
Popa-Wagner A, Carmichael ST, Kokaia Z, Kessler C, Walker LC (2007) The response of the aged brain to stroke: too much, too soon? Curr Neurovasc Res 4:216–227
Rosa MGP, Tweedale R, Elston GN (2000) Visual responses of neurons in the middle temporal area of new world monkeys after lesions of striate cortex. J Neurosci 20:5552–5563
Royet JP (1991) Stereology: a method for analyzing images. Prog Neurobiol 37:433–474
Satorre J, Cano J, Reinoso-Suárez F (1985) Stability of the neuronal population of the dorsal lateral geniculate nucleus (LGNd) of aged rats. Brain Res 339:375–377
Schmid MC, Mrowka SW, Turchi J, Saunders RC, Wilke M, Peters AJ, Ye FQ, Leopold DA (2010) Blindsight depends on the lateral geniculate nucleus. Nature 466:373–377. doi:10.1038/nature09179
Schmitz C, Hof PR (2005) Design-based stereology in neuroscience. Neuroscience 130:813–831
Sillito AM, Jones HE (2002) Corticothalamic interactions in the transfer of visual information. Philos Trans R Soc Lond B Biol Sci 357:1739–1752
Sincich LC, Park KF, Wohlgemuth MJ, Horton JC (2004) Bypassing V1: a direct geniculate input to area MT. Nat Neurosci 7:1123–1128
Solomon SG (2002) Striate cortex in dichromatic and trichromatic marmosets: neurochemical compartmentalization and geniculate input. J Comp Neurol 450:366–381
Solomon SG, Rosa MGP (2014) A simpler primate brain: the visual system of the marmoset monkey. Front Neural Circuits 8:96. doi:10.3389/fncir.2014.00096.
Vanburen JM (1963) Trans-synaptic retrograde degeneration in the visual system of primates. J Neurol Neurosurg Psych 26:402–409
Warner CE, Goldshmit Y, Bourne JA (2010) Retinal afferents synapse with relay cells targeting the middle temporal area in the pulvinar and lateral geniculate nuclei. Front Neuroanat 4:8. doi:10.3389/neuro.05.008.2010.
Weller RE, Kaas JH (1989) Parameters affecting the loss of ganglion cells of the retina following ablations of striate cortex in primates. Vis Neurosci 3:327–349
White AJ, Wilder HD, Goodchild AK, Sefton AJ, Martin PR (1998) Segregation of receptive field properties in the lateral geniculate nucleus of a New-World monkey, the marmoset Callithrix jacchus. J Neurophysiol 80:2063–2076
Williams RW, Rakic P (1988) Three-dimensional counting: an accurate and direct method to estimate numbers of cells in sectioned material. J Comp Neurol 278:344–352
Wilson JR (1993) Circuitry of the dorsal lateral geniculate nucleus in the cat and monkey. Acta Anat (Basel) 147:1–13.
Wojda U, Salinska E, Kuznicki J (2008) Calcium ions in neuronal degeneration. IUBMB Life 60:575–590. doi:10.1002/iub.91
Wolf HK, Buslei R, Schmidt-Kastner R, Schmidt-Kastner PK, Pietsch T, Wiestler OD, Blümcke I (1996) NeuN: a useful neuronal marker for diagnostic histopathology. J Histochem Cytochem 44:1167–1171
Wong-Riley MT (1972) Changes in the dorsal lateral geniculate nucleus of the squirrel monkey after unilateral ablation of the visual cortex. J Comp Neurol 146:519–548
Yin Y, Sun G, Li E, Kiselyov K, Sun D (2017) ER stress and impaired autophagy flux in neuronal degeneration and brain injury. Ageing Res Rev 34:3–14. doi:10.1016/j.arr.2016.08.008
Yu HH, Chaplin TA, Egan GW, Reser DH, Worthy KH, Rosa MG (2013) Visually evoked responses in extrastriate area MT after lesions of striate cortex in early life. J Neurosci 33:12479–12489. doi:10.1523/JNEUROSCI.0844-13.2013
Yukie M, Iwai E (1981) Direct projection from the dorsal lateral geniculate nucleus to the prestriate cortex in macaque monkeys. J Comp Neurol 201:81–97
Acknowledgements
The authors thank Rowan Tweedale for many suggestions that improved this manuscript, and acknowledge the contributions of Jonathan Chan and of the Monash Histology Platform, Department of Anatomy and Developmental Biology, for the slide scanning. Supported by research grants from the National Health and Medical Research Council (1066232, 1122220) and Australian Research Council (CE140100007, DE130100493, DE140101505).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Atapour, N., Worthy, K.H., Lui, L.L. et al. Neuronal degeneration in the dorsal lateral geniculate nucleus following lesions of primary visual cortex: comparison of young adult and geriatric marmoset monkeys. Brain Struct Funct 222, 3283–3293 (2017). https://doi.org/10.1007/s00429-017-1404-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-017-1404-4