Abstract
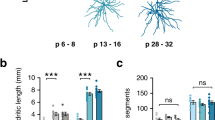
In the primate cerebral cortex, dendritic spines rapidly increase in number after birth up to infancy or mid-childhood, and then decrease towards adulthood. Abnormalities in these processes accompany several psychiatric disorders. In this study, we examined developmental changes of basal dendrites and spines of layer III pyramidal cells in the medial prefrontal cortex (mPFC) of the common marmoset. The mPFC consists of several areas with distinct features in layer organization, histochemistry, connections, and, in humans, vulnerability to psychiatric disorders. We selected three areas for examination: granular dorsomedial prefrontal (area 8B/9), dysgranular ventromedial prefrontal (area 14r), and agranular anterior cingulate (area 24) cortices. Dendritic field areas, lengths, number of branching points, and total spine number reached a peak at 2–3 postnatal months in all three areas. However, the profiles of spine formation and pruning differed across the three areas with different degrees of granularity; the amount of spine loss from the peak to adulthood was less in areas 24 (33 %) and 14r (29 %) than in area 8B/9 (43 %). Disturbance of this modest spine pruning in the less granular cortical areas may lead to an excessive loss of spines reported for areas 24 and 14r of schizophrenic patients.







Similar content being viewed by others
References
Amodio DM, Frith CD (2006) Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci 7(4):268–277
Barbas H (1995) Two prefrontal limbic systems: their common and unique features. In: Sakata H, Fuster JM (eds) Association cortex: structure and function. CRC Press, Boca Raton
Barbas H, Blatt GJ (1995) Topographically specific hippocampal projections target functionally distinct prefrontal areas in the rhesus monkey. Hippocampus 5(6):511–533
Barbas H, Pandya DN (1989) Architecture and intrinsic connections of the prefrontal cortex in the rhesus monkey. J Comp Neurol 286(3):353–375
Barbas H, Rempel-Clower N (1997) Cortical structure predicts the pattern of corticocortical connections. Cereb Cortex 7(7):635–646
Barbas H, Ghashghaei H, Dombrowski SM, Rempel-Clower NL (1999) Medial prefrontal cortices are unified by common connections with superior temporal cortices and distinguished by input from memory-related areas in the rhesus monkey. J Comp Neurol 410(3):343–367
Barros M, Tomaz C (2002) Non-human primate models for investigating fear and anxiety. Neurosci Biobehav Rev 26(2):187–201
Bennett MR (2011) Schizophrenia: susceptibility genes, dendritic-spine pathology and gray matter loss. Prog Neurobiol 95(3):275–300
Bianchi S, Stimpson CD, Duka T, Larsen MD, Janssen WG, Collins Z, Bauernfeind AL, Schapiro SJ, Baze WB, McArthur MJ, Hopkins WD, Wildman DE, Lipovich L, Kuzawa CW, Jacobs B, Hof PR, Sherwood CC (2013) Synaptogenesis and development of pyramidal neuron dendritic morphology in the chimpanzee neocortex resembles humans. Proc Natl Acad Sci USA 110(2):10395–10401
Broadbelt K, Byne W, Jones LB (2002) Evidence for a decrease in basilar dendrites of pyramidal cells in schizophrenic medial prefrontal cortex. Schizophr Res 58(1):75–81
Brodmann K (1909) Vergleichende Lokalisationslehre der Großhirnrinde. Barth, Leipzig
Buhl EH, Schlote W (1987) Intracellular lucifer yellow staining and electron microscopy of neurones in slices of fixed epitumourous human cortical tissue. Acta Neuropathol 75(2):140–146
Burkart JM, Fehr E, Efferson C, van Schaik CP (2007) Other-regarding preferences in a non-human primate: common marmosets provision food altruistically. Proc Natl Acad Sci USA 104(50):19762–19766
Burman KJ, Rosa MG (2009) Architectural subdivisions of medial and orbital frontal cortices in the marmoset monkey (Callithrix jacchus). J Comp Neurol 514(1):11–29
Cauda F, Geda E, Sacco K, D’Agata F, Duca S, Geminiani G, Keller R (2011) Grey matter abnormality in autism spectrum disorder: an activation likelihood estimation meta-analysis study. J Neurol Neurosurg Psychiatry 82(12):1304–1313
Chandolia RK, Luetjens CM, Wistuba J, Yeung CH, Nieschlag E, Simoni M (2006) Changes in endocrine profile and reproductive organs during puberty in the male marmoset monkey (Callithrix jacchus). Reproduction 132(2):355–363
Conel J (1939) The postnatal development of the human cerebral cortex, the cortex of the newborn, vol 1. Harvard University Press, Cambridge
Conel J (1941) The postnatal development of the human cerebral cortex, the cortex of the one-month infant, vol 2. Harvard University Press, Cambridge
Conel J (1947) The postnatal development of the human cerebral cortex, the cortex of the three-month infant, vol 3. Harvard University Press, Cambridge
Conel J (1951) The postnatal development of the human cerebral cortex, the cortex of the six-month infant, vol 4. Harvard University Press, Cambridge
Conel J (1955) The postnatal development of the human cerebral cortex, the cortex of the fifteen-month infant, vol 5. Harvard University Press, Cambridge
Conel J (1959) The postnatal development of the human cerebral cortex, the cortex of the twenty-four-month infant, vol 6. Harvard University Press, Cambridge
Conel J (1963) The postnatal development of the human cerebral cortex, the cortex of the four-year child, vol 7. Harvard University Press, Cambridge
Conel J (1967) The postnatal development of the human cerebral cortex, the cortex of the six-year child, vol 8. Harvard University Press, Cambridge
Dombrowski SM, Barbas H (1996) Differential expression of NADPH diaphorase in functionally distinct prefrontal cortices in the rhesus monkey. Neuroscience 72:49–62
Dombrowski SM, Hilgetag CC, Barbas H (2001) Quantitative architecture distinguishes prefrontal cortical systems in the rhesus monkey. Cereb Cortex 11:975–988
Eayrs JT, Goodhead B (1959) Postnatal development of the cerebral cortex in the rat. J Anat 93:385–402
Ebert DH, Greenberg ME (2013) Activity-dependent neuronal signaling and autism spectrum disorder. Nature 493(7432):327–337
Elston GN (2001) Interlaminar differences in the pyramidal cell phenotype in cortical areas 7 m and STP (the superior temporal polysensory area) of the macaque monkey. Exp Brain Res 138(2):141–152
Elston GN, Fujita I (2014) Pyramidal cell development: postnatal spinogenesis, dendritic growth, axon growth, and electrophysiology. Front Neuroanat (in press)
Elston GN, Rosa MGP (1997) The occipitoparietal pathway of the macaque monkey: comparison of pyramidal cell morphology in the layer III of functionally related cortical visual areas. Cereb Cortex 7(5):432–452
Elston GN, Benavides-Piccione R, DeFelipe J (2005a) A study of pyramidal cell structure in the cingulate cortex of the macaque monkey with comparative notes on inferotemporal and primary visual cortex. Cereb Cortex 15(1):64–73
Elston GN, Benavides-Piccione R, Elston A, DeFelipe J, Manger P (2005b) Specialization in pyramidal cell structure in the cingulate cortex of the Chacma baboon (Papio ursinus): an intracellular injection study of the posterior and anterior cingulate gyrus with comparative notes on the macaque and vervet monkeys. Neurosci Lett 387(3):130–135
Elston GN, Benavides-Piccione R, Elston A, Manger P, DeFelipe J (2005c) Regional specialization in pyramidal cell structure in the limbic cortex of the vervet monkey (Cercopithecus pygerythrus): an intracellular injection study of the anterior and posterior cingulate gyrus. Exp Brain Res 167(3):315–323
Elston GN, Oga T, Fujita I (2009) Spinogenesis and pruning scales across functional hierarchies. J Neurosci 29(10):3271–3275
Elston GN, Oga T, Okamoto T, Fujita I (2010a) Spinogenesis and pruning from early visual onset to adulthood: an intracellular injection study of layer III pyramidal cells in the ventral visual cortical pathway of the macaque monkey. Cereb Cortex 20(6):1398–1408
Elston GN, Oga T, Okamoto T, Fujita I (2010b) Spinogenesis and pruning in the primary auditory cortex of the macaque monkey (Macaca fascicularis): an intracellular injection study of layer III pyramidal cells. Brain Res 1316:36–42
Elston GN, Oga T, Okamoto T, Fujita I (2011) Spinogenesis and pruning in the anterior ventral inferotemporal cortex of the macaque monkey: an intracellular injection study of layer III pyramidal cells. Front Neuroanat 5:42
Fukuoka T, Sumida K, Yamada T, Higuchi C, Nakagaki K, Nakamura K, Kohsaka S, Saito K, Oeda K (2010) Gene expression profiles in the common marmoset brain determined using a newly developed common marmoset-specific DNA microarray. Neurosci Res 66(1):62–85
Giguere M, Goldman-Rakic PS (1988) Mediodorsal nucleus: areal, laminar, and tangential distribution of afferents and efferents in the frontal lobe of rhesus monkeys. J Comp Neurol 277(2):195–213
Glantz LA, Lewis DA (2000) Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry 57(1):65–73
Glausier JR, Lewis DA (2013) Dendritic spine pathology in schizophrenia. Neuroscience 22(251):90–107
Gray EG (1959) Electron microscopy of synaptic contacts on dendritic spines of the cerebral cortex. Nature 183(4675):1592–1594
Hustler JJ, Zhang H (2010) Increased dendritic spine densities on cortical projection neurons in autism spectrum disorders. Brain Res 1309:83–94
Huttenlocher PR (1990) Morphometric study of human cerebral cortex development. Neuropsychologia 28(6):517–527
Huttenlocher PR, Dabholkar AS (1997) Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol 387(2):167–178
Jacobs B, Scheibel AB (2002) Regional dendritic variation in primate cortical pyramidal cells. In: Schüz A, Miller R (eds) Cortical areas: unity and diversity. Taylor and Francis, London
Jacobs B, Driscoll L, Schall M (1997) Life-span dendritic and spine changes in areas 10 and 18 of human cortex: a quantitative Golgi study. J Comp Neurol 386:661–680
Jacobs B, Schall M, Prather M, Kapler E, Driscoll L, Baca S, Jacobs J, Ford K, Wainwright M, Treml M (2001) Regional dendritic and spine variation in human cerebral cortex: a quantitative study. Cereb Cortex 11:558–571
Kalus P, Muller TJ, Zuschratter W, Seniz D (2000) The dendritic architecture of prefrontal pyramidal neurons in schizophrenic patients. NeuroReport 11(16):3621–3625
Kaufmann WE, Moser HW (2000) Dendritic anomalies in disorders associated with mental retardation. Cereb Cortex 10(10):981–991
Larsen M, Bjarkam CR, Stoltenberg M, Sørensen JC, Danscher G (2003) An autometallographic technique for myelin staining in formaldehyde-fixed tissue. Histol Histopathol 18(4):1125–1130
Levenga J, Willemsen R (2012) Perturbation of dendritic protrusions in intellectual disability. Prog Brain Res 197:153–168
Malach R (1994) Cortical columns as devices for maximizing neuronal diversity. Trends Neurosci 17:101–104
McKinnell C, Saunders PT, Fraser HM, Kelnar CJ, Kivlin C, Morris KD, Sharpe RM (2001) Comparison of androgen receptor and estrogen receptor beta immunoexpression in the testes of the common marmoset (Callithrix jacchus) from birth to adulthood: low androgen receptor immunoexpression in Sertoli cells during the neonatal increase in testosterone concentrations. Reproduction 122(3):419–429
Missler M, Wolff A, Merker HJ, Wolff JR (1993) Pre- and postnatal development of the primary visual cortex of the common marmoset. II. Formation, remodelling, and elimination of synapses as overlapping processes. J Comp Neurol 333(1993):53–67
Nestor MW, Hoffman DA (2012) Aberrant dendritic excitability: a common pathophysiology in CNS disorders affecting memory? Mol Neurobiol 45(3):478–487
Oga T, Aoi T, Sasaki T, Fujita I, Ichinohe N (2013) Postnatal development of layer III pyramidal cells in the primary visual, inferior temporal, and prefrontal cortices of the marmoset. Front Neural Circuits 7:31
Ongür D, Price JL (2000) The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex 10(3):206–219
Paxinos G, Watson C, Petrides M, Rosa MGP, Tokuno H (2011) The marmoset brain in stereotaxic coordinates. Academic Press, New York
Penzes P, Cahill ME, Jones KA, VanLeeuwen JE, Woolfrey KM (2011) Dendritic spine pathology in neuropsychiatric disorders. Nat Neurosci 14(3):285–293
Petanjek Z, Judas M, Kostović I, Uylings HB (2008) Lifespan alterations of basal dendritic trees of pyramidal neurons in the human prefrontal cortex: a layer-specific pattern. Cereb Cortex 18(4):915–929
Petanjek Z, Judaš M, Šimic G, Rasin MR, Uylings HB, Rakic P, Kostovic I (2011) Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci USA 108(32):13281–13286
Poirazi P, Mel BW (2001) Impact of active dendrites and structural plasticity on the memory capacity of neural tissue. Neuron 29(3):779–796
Saleem KS, Suzuki W, Tanaka K, Hashikawa T (2000) Connections between anterior inferotemporal cortex and superior temporal sulcus regions in the macaque monkey. J Neurosci 20(13):5083–5101
Sasaki T, Oga T, Nakagaki K, Sakai K, Sumida K, Hoshino K, Miyawaki I, Saito K, Suto F, Ichinohe N (2014a) Developmental expression profiles of axon guidance signaling and the immune system in the marmoset cortex: potential molecular mechanisms of pruning of dendritic spines during primate synapse formation in late infancy and prepuberty (I). Biochem Biophys Res Commun 444(3):302–306
Sasaki T, Oga T, Nakagaki K, Sakai K, Sumida K, Hoshino K, Miyawaki I, Saito K, Suto F, Ichinohe N (2014b) Developmental genetic profiles of glutamate receptor system, neuromodulator system, protector of normal tissue and mitochondria, and reelin in marmoset cortex: potential molecular mechanisms of pruning phase of spines in primate synaptic formation process during the end of infancy and prepuberty (II). Biochem Biophys Res Commun 444(3):307–310
Sholl DA (1953) Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat 87(4):387–406
Stuart G, Spruston N, Hausser M (2008) Dendrites, 2nd edn. Oxford University Press, Oxford
Suzuki WA, Amaral DG (2003) Where are the perirhinal and parahippocampal cortices? A historical overview of the nomenclature and the boundaries applied to the primate medial temporal lobe. Neuroscience 120(4):893–906
Travis K, Ford K, Jacobs B (2005) Regional dendritic variation in neonatal human cortex: a quantitative Golgi study. Dev Neurosci 27(5):277–287
Valverde F (1967) Apical dendritic spines of the visual cortex and light deprivation in the mouse. Exp Brain Res 3(4):337–352
van Spronsen M, Hoogenraad CC (2010) Synapse pathology in psychiatric and neurologic disease. Curr Neurol Neurosci Rep 10(3):207–214
Yuste R (2010) Dendritic spines. MIT Press, Cambridge
Acknowledgments
This work was supported by an Intramural Research Grant (grant number 23-7) for Neurological and Psychiatric Disorders from the National Center of Neurology and Psychiatry, by a Funding Program for World-Leading Innovative R&D on Science and Technology (FIRST Program), by the Strategic Research Program for Brain Science, by a Grant-in-Aid for Scientific Research on Innovative Areas “Shitsukan” (No. 22135007), “Foundation of Synapse Neurocircuit Pathology” (No. 25110740), “Glia-Assembly” (No. 25117001), and “Seishun-no” (No. 26118717) from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT), Japan (to N.I.), and by grants (Nos. 23240047 and 23135522) from MEXT, Japan (to I.F). T.O. was supported by a Grant-in-Aid for Scientific Research on Innovative Areas (Comprehensive Brain Science Network) from MEXT, Japan. We thank Takako Suzuki, Tomoko Manabe, and Dr. Taku Banno of the National Center of Neurology and Psychiatry for their technical assistance. We also thank Dr. Keiko Nakagaki of the National Center of Neurology and Psychiatry and Yu Nabeshima and Ryoichi Saito of CLEA Japan, Inc. for their support in the conduct of experiments.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationship that could be construed as a potential conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
T. Sasaki and H. Aoi contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sasaki, T., Aoi, H., Oga, T. et al. Postnatal development of dendritic structure of layer III pyramidal neurons in the medial prefrontal cortex of marmoset. Brain Struct Funct 220, 3245–3258 (2015). https://doi.org/10.1007/s00429-014-0853-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-014-0853-2