Abstract
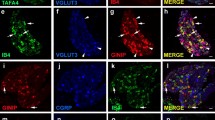
In recent pain studies on animal models, α7 nicotinic acetylcholine receptor (nAChR) agonists demonstrated analgesic, anti-hyperalgesic and anti-inflammatory effects, apparently acting through some peripheral receptors. Assuming possible involvement of α7 nAChRs on nociceptive sensory neurons, we investigated the morphological and neurochemical features of the α7 nAChR-expressing subpopulation of dorsal root ganglion (DRG) neurons and their ability to transport α7 nAChR axonally. In addition, α7 receptor activity and its putative role in pain signal neurotransmitter release were studied. Medium-sized α7 nAChR-expressing neurons prevailed, although the range covered all cell sizes. These cells accounted for one-fifth of total medium and large DRG neurons and <5 % of small ones. 83.2 % of α7 nAChR-expressing DRG neurons were peptidergic nociceptors (CGRP-immunopositive), one half of which had non-myelinated C-fibers and the other half had myelinated Aδ- and likely Aα/β-fibers, whereas 15.2 % were non-peptidergic C-fiber nociceptors binding isolectin B4. All non-peptidergic and a third of peptidergic α7 nAChR-bearing nociceptors expressed TRPV1, a capsaicin-sensitive noxious stimulus transducer. Nerve crush experiments demonstrated that CGRPergic DRG nociceptors axonally transported α7 nAChRs both to the spinal cord and periphery. α7 nAChRs in DRG neurons were functional as their specific agonist PNU282987 evoked calcium rise enhanced by α7-selective positive allosteric modulator PNU120596. However, α7 nAChRs do not modulate neurotransmitter CGRP and glutamate release from DRG neurons since nicotinic ligands affected neither their basal nor provoked levels, showing the necessity of further studies to elucidate the true role of α7 nAChRs in those neurons.








Similar content being viewed by others
References
Abdin MJ, Morioka N, Morita K, Kitayama T, Kitayama S, Nakashima T, Dohi T (2006) Analgesic action of nicotine on tibial nerve transection (TNT)-induced mechanical allodynia through enhancement of the glycinergic inhibitory system in spinal cord. Life Sci 80(1):9–16. doi:10.1016/j.lfs.2006.08.011
Bennett DL, Averill S, Clary DO, Priestley JV, McMahon SB (1996) Postnatal changes in the expression of the trkA high-affinity NGF receptor in primary sensory neurons. Eur J Neurosci 8(10):2204–2208
Berg DK, Conroy WG (2002) Nicotinic alpha 7 receptors: synaptic options and downstream signaling in neurons. J Neurobiol 53(4):512–523. doi:10.1002/neu.10116
Bernardini N, Sauer SK, Haberberger R, Fischer MJ, Reeh PW (2001) Excitatory nicotinic and desensitizing muscarinic (M2) effects on C-nociceptors in isolated rat skin. J Neurosci 21(9):3295–3302 (pii: 21/9/3295)
Bodnar AL, Cortes-Burgos LA, Cook KK, Dinh DM, Groppi VE, Hajos M, Higdon NR, Hoffmann WE, Hurst RS, Myers JK, Rogers BN, Wall TM, Wolfe ML, Wong E (2005) Discovery and structure-activity relationship of quinuclidine benzamides as agonists of alpha7 nicotinic acetylcholine receptors. J Med Chem 48(4):905–908. doi:10.1021/jm049363q
Boyd RT, Jacob MH, McEachern AE, Caron S, Berg DK (1991) Nicotinic acetylcholine receptor mRNA in dorsal root ganglion neurons. J Neurobiol 22(1):1–14. doi:10.1002/neu.480220102
Bracci-Laudiero L, Aloe L, Buanne P, Finn A, Stenfors C, Vigneti E, Theodorsson E, Lundeberg T (2002) NGF modulates CGRP synthesis in human B-lymphocytes: a possible anti-inflammatory action of NGF? J Neuroimmunol 123(1–2):58–65 (pii: S0165572801004751)
Brenneis C, Kistner K, Puopolo M, Segal D, Roberson D, Sisignano M, Labocha S, Ferreiros N, Strominger A, Cobos EJ, Ghasemlou N, Geisslinger G, Reeh PW, Bean BP, Woolf CJ (2013) Phenotyping the function of TRPV1-expressing sensory neurons by targeted axonal silencing. J Neurosci 33(1):315–326. doi:10.1523/JNEUROSCI.2804-12.2013
Carr RW, Proske U (1996) Action of cholinesters on sensory nerve endings in skin and muscle. Clin Exp Pharmacol Physiol 23(5):355–362
Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389(6653):816–824. doi:10.1038/39807
Cuevas J, Roth AL, Berg DK (2000) Two distinct classes of functional 7-containing nicotinic receptor on rat superior cervical ganglion neurons. J Physiol 525(Pt 3):735–746 (pii: PHY_0374)
Dajas-Bailador F, Wonnacott S (2004) Nicotinic acetylcholine receptors and the regulation of neuronal signalling. Trends Pharmacol Sci 25(6):317–324. doi:10.1016/j.tips.2004.04.006
Damaj MI, Meyer EM, Martin BR (2000) The antinociceptive effects of alpha7 nicotinic agonists in an acute pain model. Neuropharmacology 39(13):2785–2791 (pii: S0028390800001398)
Di Angelantonio S, Giniatullin R, Costa V, Sokolova E, Nistri A (2003) Modulation of neuronal nicotinic receptor function by the neuropeptides CGRP and substance P on autonomic nerve cells. Br J Pharmacol 139(6):1061–1073. doi:10.1038/sj.bjp.0705337
Djouhri L, Lawson SN (2004) Abeta-fiber nociceptive primary afferent neurons: a review of incidence and properties in relation to other afferent A-fiber neurons in mammals. Brain Res Brain Res Rev 46(2):131–145. doi:10.1016/j.brainresrev.2004.07.015
Drisdel RC, Manzana E, Green WN (2004) The role of palmitoylation in functional expression of nicotinic alpha7 receptors. J Neurosci 24(46):10502–10510. doi:10.1523/JNEUROSCI.3315-04.2004
Fang X, Djouhri L, McMullan S, Berry C, Waxman SG, Okuse K, Lawson SN (2006) Intense isolectin-B4 binding in rat dorsal root ganglion neurons distinguishes C-fiber nociceptors with broad action potentials and high Nav1.9 expression. J Neurosci 26(27):7281–7292. doi:10.1523/JNEUROSCI.1072-06.2006
Feuerbach D, Lingenhoehl K, Olpe HR, Vassout A, Gentsch C, Chaperon F, Nozulak J, Enz A, Bilbe G, McAllister K, Hoyer D (2009) The selective nicotinic acetylcholine receptor alpha7 agonist JN403 is active in animal models of cognition, sensory gating, epilepsy and pain. Neuropharmacology 56(1):254–263. doi:10.1016/j.neuropharm.2008.08.025
Fornaro M, Lee JM, Raimondo S, Nicolino S, Geuna S, Giacobini-Robecchi M (2008) Neuronal intermediate filament expression in rat dorsal root ganglia sensory neurons: an in vivo and in vitro study. Neuroscience 153(4):1153–1163. doi:10.1016/j.neuroscience.2008.02.080
Franco-Cereceda A, Saria A, Lundberg JM (1989) Differential release of calcitonin gene-related peptide and neuropeptide Y from the isolated heart by capsaicin, ischaemia, nicotine, bradykinin and ouabain. Acta Physiol Scand 135(2):173–187
Franco-Cereceda A, Rydh M, Dalsgaard CJ (1992) Nicotine- and capsaicin-, but not potassium-evoked CGP-release from cultured guinea-pig spinal ganglia is inhibited by Ruthenium red. Neurosci Lett 137(1):72–74
Fucile S, Sucapane A, Eusebi F (2005) Ca2+ permeability of nicotinic acetylcholine receptors from rat dorsal root ganglion neurones. J Physiol 565(Pt 1):219–228. doi:10.1113/jphysiol.2005.084871
Genzen JR, McGehee DS (2003) Short- and long-term enhancement of excitatory transmission in the spinal cord dorsal horn by nicotinic acetylcholine receptors. Proc Natl Acad Sci USA 100(11):6807–6812. doi:10.1073/pnas.1131709100
Genzen JR, Van Cleve W, McGehee DS (2001) Dorsal root ganglion neurons express multiple nicotinic acetylcholine receptor subtypes. J Neurophysiol 86(4):1773–1782
Gergalova G, Lykhmus O, Kalashnyk O, Koval L, Chernyshov V, Kryukova E, Tsetlin V, Komisarenko S, Skok M (2012) Mitochondria express alpha7 nicotinic acetylcholine receptors to regulate Ca2+ accumulation and cytochrome c release: study on isolated mitochondria. PLoS ONE 7(2):e31361. doi:10.1371/journal.pone.0031361
Gibson SJ, Polak JM, Bloom SR, Sabate IM, Mulderry PM, Ghatei MA, McGregor GP, Morrison JF, Kelly JS, Evans RM et al (1984) Calcitonin gene-related peptide immunoreactivity in the spinal cord of man and of eight other species. J Neurosci 4(12):3101–3111
Gold MS (2012) Whole-cell recording in isolated primary sensory neurons. Methods Mol Biol 851:73–97. doi:10.1007/978-1-61779-561-9_5
Gotti C, Clementi F (2004) Neuronal nicotinic receptors: from structure to pathology. Prog Neurobiol 74(6):363–396. doi:10.1016/j.pneurobio.2004.09.006
Gotti C, Zoli M, Clementi F (2006) Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharmacol Sci 27(9):482–491. doi:10.1016/j.tips.2006.07.004
Gotti C, Clementi F, Fornari A, Gaimarri A, Guiducci S, Manfredi I, Moretti M, Pedrazzi P, Pucci L, Zoli M (2009) Structural and functional diversity of native brain neuronal nicotinic receptors. Biochem Pharmacol 78(7):703–711. doi:10.1016/j.bcp.2009.05.024
Gray R, Rajan AS, Radcliffe KA, Yakehiro M, Dani JA (1996) Hippocampal synaptic transmission enhanced by low concentrations of nicotine. Nature 383(6602):713–716. doi:10.1038/383713a0
Gundisch D, Eibl C (2011) Nicotinic acetylcholine receptor ligands, a patent review (2006–2011). Expert Opin Ther Pat 21(12):1867–1896. doi:10.1517/13543776.2011.637919
Guo A, Vulchanova L, Wang J, Li X, Elde R (1999) Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur J Neurosci 11(3):946–958
Gurun MS, Parker R, Eisenach JC, Vincler M (2009) The effect of peripherally administered CDP-choline in an acute inflammatory pain model: the role of alpha7 nicotinic acetylcholine receptor. Anesth Analg 108(5):1680–1687. doi:10.1213/ane.0b013e31819dcd08
Haberberger RV, Bernardini N, Kress M, Hartmann P, Lips KS, Kummer W (2004) Nicotinic acetylcholine receptor subtypes in nociceptive dorsal root ganglion neurons of the adult rat. Auton Neurosci 113(1–2):32–42. doi:10.1016/j.autneu.2004.05.008
Hua XY, Jinno S, Back SM, Tam EK, Yaksh TL (1994) Multiple mechanisms for the effects of capsaicin, bradykinin and nicotine on CGRP release from tracheal afferent nerves: role of prostaglandins, sympathetic nerves and mast cells. Neuropharmacology 33(10):1147–1154
Hua XY, Wong S, Jinno S, Yaksh TL (1995) Pharmacology of calcitonin gene related peptide release from sensory terminals in the rat trachea. Can J Physiol Pharmacol 73(7):999–1006
Hurst RS, Hajos M, Raggenbass M, Wall TM, Higdon NR, Lawson JA, Rutherford-Root KL, Berkenpas MB, Hoffmann WE, Piotrowski DW, Groppi VE, Allaman G, Ogier R, Bertrand S, Bertrand D, Arneric SP (2005) A novel positive allosteric modulator of the alpha7 neuronal nicotinic acetylcholine receptor: in vitro and in vivo characterization. J Neurosci 25(17):4396–4405. doi:10.1523/JNEUROSCI.5269-04.2005
Huston JM (2012) The vagus nerve and the inflammatory reflex: wandering on a new treatment paradigm for systemic inflammation and sepsis. Surg Infect (Larchmt) 13(4):187–193. doi:10.1089/sur.2012.126
Jiang L, Role LW (2008) Facilitation of cortico-amygdala synapses by nicotine: activity-dependent modulation of glutamatergic transmission. J Neurophysiol 99(4):1988–1999. doi:10.1152/jn.00933.2007
Julius D, Basbaum AI (2001) Molecular mechanisms of nociception. Nature 413(6852):203–210. doi:10.1038/35093019
Kashihara Y, Sakaguchi M, Kuno M (1989) Axonal transport and distribution of endogenous calcitonin gene-related peptide in rat peripheral nerve. J Neurosci 9(11):3796–3802
Kawasaki H, Takatori S, Zamami Y, Koyama T, Goda M, Hirai K, Tangsucharit P, Jin X, Hobara N, Kitamura Y (2011) Paracrine control of mesenteric perivascular axo-axonal interaction. Acta Physiol (Oxf) 203(1):3–11. doi:10.1111/j.1748-1716.2010.02197.x
Kobayashi K, Fukuoka T, Obata K, Yamanaka H, Dai Y, Tokunaga A, Noguchi K (2005) Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J Comp Neurol 493(4):596–606. doi:10.1002/cne.20794
Kukhtina VV, Weise K, Osipov AV, Starkov VG, Titov MI, Esipov SE, Ovchinnikova TV, Tsetlin VI, Utkin IuN (2000) MALDI-mass spectrometry for identification of new proteins in snake venoms. Bioorg Khim 26(11):803–807
Kummer W (1994) Sensory ganglia as a target of autonomic and sensory nerve fibres in the guinea-pig. Neuroscience 59(3):739–754
Lawson SN (2002) Phenotype and function of somatic primary afferent nociceptive neurones with C-Adelta- or Aalpha/beta-fibres. Exp Physiol 87(2):239–244 (pii: EPH_2350)
Lawson SN, Crepps B, Perl ER (2002) Calcitonin gene-related peptide immunoreactivity and afferent receptive properties of dorsal root ganglion neurones in guinea-pigs. J Physiol 540(Pt 3):989–1002 (pii: PHY_13086)
Liu T, Fujita T, Kumamoto E (2011) Acetylcholine and norepinephrine mediate GABAergic but not glycinergic transmission enhancement by melittin in adult rat substantia gelatinosa neurons. J Neurophysiol 106(1):233–246. doi:10.1152/jn.00838.2010
Liu Y, Hu J, Wu J, Zhu C, Hui Y, Han Y, Huang Z, Ellsworth K, Fan W (2012) alpha7 nicotinic acetylcholine receptor-mediated neuroprotection against dopaminergic neuron loss in an MPTP mouse model via inhibition of astrocyte activation. J Neuroinflamm 9:98. doi:10.1186/1742-2094-9-98
Lou YP, Franco-Cereceda A, Lundberg JM (1992) Different ion channel mechanisms between low concentrations of capsaicin and high concentrations of capsaicin and nicotine regarding peptide release from pulmonary afferents. Acta Physiol Scand 146(1):119–127
Marchi M, Grilli M (2010) Presynaptic nicotinic receptors modulating neurotransmitter release in the central nervous system: functional interactions with other coexisting receptors. Prog Neurobiol 92(2):105–111. doi:10.1016/j.pneurobio.2010.06.004
Marrero MB, Bencherif M, Lippiello PM, Lucas R (2011) Application of alpha7 nicotinic acetylcholine receptor agonists in inflammatory diseases: an overview. Pharm Res 28(2):413–416. doi:10.1007/s11095-010-0283-7
McGehee DS, Heath MJ, Gelber S, Devay P, Role LW (1995) Nicotine enhancement of fast excitatory synaptic transmission in CNS by presynaptic receptors. Science 269(5231):1692–1696
Medhurst SJ, Hatcher JP, Hille CJ, Bingham S, Clayton NM, Billinton A, Chessell IP (2008) Activation of the alpha7-nicotinic acetylcholine receptor reverses complete freund adjuvant-induced mechanical hyperalgesia in the rat via a central site of action. J Pain 9(7):580–587. doi:10.1016/j.jpain.2008.01.336
Millington WR, Aizenman E, Bierkamper GG, Zarbin MA, Kuhar MJ (1985) Axonal transport of alpha-bungarotoxin binding sites in rat sciatic nerve. Brain Res 340(2):269–276 (pii: 0006-8993(85)90923-0)
Mitchell K, Bates BD, Keller JM, Lopez M, Scholl L, Navarro J, Madian N, Haspel G, Nemenov MI, Iadarola MJ (2010) Ablation of rat TRPV1-expressing Adelta/C-fibers with resiniferatoxin: analysis of withdrawal behaviors, recovery of function and molecular correlates. Mol Pain 6:94. doi:10.1186/1744-8069-6-94
Molliver DC, Wright DE, Leitner ML, Parsadanian AS, Doster K, Wen D, Yan Q, Snider WD (1997) IB4-binding DRG neurons switch from NGF to GDNF dependence in early postnatal life. Neuron 19(4):849–861 (pii: S0896-6273(00)80966-6)
Murray TA, Bertrand D, Papke RL, George AA, Pantoja R, Srinivasan R, Liu Q, Wu J, Whiteaker P, Lester HA, Lukas RJ (2012) alpha7beta2 nicotinic acetylcholine receptors assemble, function, and are activated primarily via their alpha7-alpha7 interfaces. Mol Pharmacol 81(2):175–188. doi:10.1124/mol.111.074088
Nassenstein C, Taylor-Clark TE, Myers AC, Ru F, Nandigama R, Bettner W, Undem BJ (2010) Phenotypic distinctions between neural crest and placodal derived vagal C-fibres in mouse lungs. J Physiol 588(Pt 23):4769–4783. doi:10.1113/jphysiol.2010.195339
Ninkovic M, Hunt SP (1983) Alpha-bungarotoxin binding sites on sensory neurones and their axonal transport in sensory afferents. Brain Res 272(1):57–69 (pii: 0006-8993(83)90364-5)
Nizri E, Brenner T (2011) Modulation of inflammatory pathways by the immune cholinergic system. Amino Acids. doi:10.1007/s00726-011-1192-8
Olofsson PS, Rosas-Ballina M, Levine YA, Tracey KJ (2012) Rethinking inflammation: neural circuits in the regulation of immunity. Immunol Rev 248(1):188–204. doi:10.1111/j.1600-065X.2012.01138.x
Pacini A, Di Cesare Mannelli L, Bonaccini L, Ronzoni S, Bartolini A, Ghelardini C (2010) Protective effect of alpha7 nAChR: behavioural and morphological features on neuropathy. Pain 150(3):542–549. doi:10.1016/j.pain.2010.06.014
Parada E, Egea J, Buendia I, Negredo P, Cunha AC, Cardoso S, Soares MP, Lopez MG (2013) The microglial alpha7 acetylcholine nicotinic receptor is a key element in promoting neuroprotection by inducing HO-1 via Nrf2. Antioxid Redox Signal. doi:10.1089/ars.2012.4671
Price TJ, Flores CM (2007) Critical evaluation of the colocalization between calcitonin gene-related peptide, substance P, transient receptor potential vanilloid subfamily type 1 immunoreactivities, and isolectin B4 binding in primary afferent neurons of the rat and mouse. J Pain 8(3):263–272. doi:10.1016/j.jpain.2006.09.005
Putz G, Kristufek D, Orr-Urtreger A, Changeux JP, Huck S, Scholze P (2008) Nicotinic acetylcholine receptor-subunit mRNAs in the mouse superior cervical ganglion are regulated by development but not by deletion of distinct subunit genes. J Neurosci Res 86(5):972–981. doi:10.1002/jnr.21559
Rau KK, Johnson RD, Cooper BY (2005) Nicotinic AChR in subclassified capsaicin-sensitive and -insensitive nociceptors of the rat DRG. J Neurophysiol 93(3):1358–1371. doi:10.1152/jn.00591.2004
Ribeiro-da-Silva A, Cuello AC (1990) Choline acetyltransferase-immunoreactive profiles are presynaptic to primary sensory fibers in the rat superficial dorsal horn. J Comp Neurol 295(3):370–384. doi:10.1002/cne.902950303
Richardson JD, Vasko MR (2002) Cellular mechanisms of neurogenic inflammation. J Pharmacol Exp Ther 302(3):839–845. doi:10.1124/jpet.102.032797
Ringkamp M, Peng YB, Wu G, Hartke TV, Campbell JN, Meyer RA (2001) Capsaicin responses in heat-sensitive and heat-insensitive A-fiber nociceptors. J Neurosci 21(12):4460–4468
Roth AL, Shoop RD, Berg DK (2000) Targeting alpha7-containing nicotinic receptors on neurons to distal locations. Eur J Pharmacol 393(1–3):105–112 (pii: S0014-2999(00)00092-3)
Rowley TJ, McKinstry A, Greenidge E, Smith W, Flood P (2010) Antinociceptive and anti-inflammatory effects of choline in a mouse model of postoperative pain. Br J Anaesth 105(2):201–207. doi:10.1093/bja/aeq113
Shelukhina IV, Kryukova EV, Lips KS, Tsetlin VI, Kummer W (2009) Presence of alpha7 nicotinic acetylcholine receptors on dorsal root ganglion neurons proved using knockout mice and selective alpha-neurotoxins in histochemistry. J Neurochem 109(4):1087–1095. doi:10.1111/j.1471-4159.2009.06033.x
Shen JX, Yakel JL (2009) Nicotinic acetylcholine receptor-mediated calcium signaling in the nervous system. Acta Pharmacol Sin 30(6):673–680. doi:10.1038/aps.2009.64
Shen JX, Yakel JL (2012) Functional alpha7 nicotinic ACh receptors on astrocytes in rat hippocampal CA1 slices. J Mol Neurosci 48(1):14–21. doi:10.1007/s12031-012-9719-3
Shytle RD, Mori T, Townsend K, Vendrame M, Sun N, Zeng J, Ehrhart J, Silver AA, Sanberg PR, Tan J (2004) Cholinergic modulation of microglial activation by alpha 7 nicotinic receptors. J Neurochem 89(2):337–343. doi:10.1046/j.1471-4159.2004.02347.x
Si ML, Lee TJ (2003) Pb2+ inhibition of sympathetic alpha 7-nicotinic acetylcholine receptor-mediated nitrergic neurogenic dilation in porcine basilar arteries. J Pharmacol Exp Ther 305(3):1124–1131. doi:10.1124/jpet.102.046854
Steen KH, Reeh PW (1993) Actions of cholinergic agonists and antagonists on sensory nerve endings in rat skin, in vitro. J Neurophysiol 70(1):397–405
Sucher NJ, Cheng TP, Lipton SA (1990) Neural nicotinic acetylcholine responses in sensory neurons from postnatal rat. Brain Res 533(2):248–254 (pii: 0006-8993(90)91346-I)
Thompson RJ, Doran JF, Jackson P, Dhillon AP, Rode J (1983) PGP 9.5—a new marker for vertebrate neurons and neuroendocrine cells. Brain Res 278(1–2):224–228 (pii: 0006-8993(83)90241-X)
Thomsen MS, Mikkelsen JD (2012) The alpha7 nicotinic acetylcholine receptor ligands methyllycaconitine, NS6740 and GTS-21 reduce lipopolysaccharide-induced TNF-alpha release from microglia. J Neuroimmunol 251(1–2):65–72. doi:10.1016/j.jneuroim.2012.07.006
Thomsen MS, Hansen HH, Timmerman DB, Mikkelsen JD (2010) Cognitive improvement by activation of alpha7 nicotinic acetylcholine receptors: from animal models to human pathophysiology. Curr Pharm Des 16(3):323–343
Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D (1998) The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 21(3):531–543 (pii: S0896-6273(00)80564-4)
Tsetlin V, Utkin Y, Kasheverov I (2009) Polypeptide and peptide toxins, magnifying lenses for binding sites in nicotinic acetylcholine receptors. Biochem Pharmacol 78(7):720–731. doi:10.1016/j.bcp.2009.05.032
Uteshev VV (2012) alpha7 nicotinic ACh receptors as a ligand-gated source of Ca(2+) ions: the search for a Ca(2+) optimum. Adv Exp Med Biol 740:603–638. doi:10.1007/978-94-007-2888-2_27
Utkin YN, Kukhtina VV, Kryukova EV, Chiodini F, Bertrand D, Methfessel C, Tsetlin VI (2001) “Weak toxin” from Naja kaouthia is a nontoxic antagonist of alpha 7 and muscle-type nicotinic acetylcholine receptors. J Biol Chem 276(19):15810–15815. doi:10.1074/jbc.M100788200
Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Yang H, Ulloa L, Al-Abed Y, Czura CJ, Tracey KJ (2003) Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 421(6921):384–388. doi:10.1038/nature01339
Wang Y, Su DM, Wang RH, Liu Y, Wang H (2005) Antinociceptive effects of choline against acute and inflammatory pain. Neuroscience 132(1):49–56. doi:10.1016/j.neuroscience.2004.12.026
Ween H, Thorin-Hagene K, Andersen E, Gronlien JH, Lee CH, Gopalakrishnan M, Malysz J (2010) Alpha3* and alpha 7 nAChR-mediated Ca2+ transient generation in IMR-32 neuroblastoma cells. Neurochem Int 57(3):269–277. doi:10.1016/j.neuint.2010.06.005
Wetsel WC (2011) Sensing hot and cold with TRP channels. Int J Hyperth 27(4):388–398. doi:10.3109/02656736.2011.554337
Wonnacott S, Barik J, Dickinson J, Jones IW (2006) Nicotinic receptors modulate transmitter cross talk in the CNS: nicotinic modulation of transmitters. J Mol Neurosci 30(1–2):137–140. doi:10.1385/JMN:30:1:137
Woolf CJ, Ma Q (2007) Nociceptors—noxious stimulus detectors. Neuron 55(3):353–364. doi:10.1016/j.neuron.2007.07.016
Wu J, Lukas RJ (2011) Naturally-expressed nicotinic acetylcholine receptor subtypes. Biochem Pharmacol 82(8):800–807. doi:10.1016/j.bcp.2011.07.067
Zhang X, Bao L, Xu ZQ, Kopp J, Arvidsson U, Elde R, Hokfelt T (1994) Localization of neuropeptide Y Y1 receptors in the rat nervous system with special reference to somatic receptors on small dorsal root ganglion neurons. Proc Natl Acad Sci USA 91(24):11738–11742
Zoli M, Le Novere N, Hill JA Jr, Changeux JP (1995) Developmental regulation of nicotinic ACh receptor subunit mRNAs in the rat central and peripheral nervous systems. J Neurosci 15(3 Pt 1):1912–1939
Acknowledgments
This work was supported by the LOEWE Program of the State of Hessen, Research Focus Non-neuronal cholinergic system, by grants of Russian Foundation for Basic Research No. 13-04-40377-N KOMFI and MCB RAS program. We thank Martin Bodenbenner, Tamara Papadakis, Silke Wiegand and Anna Goldenberg for technical help, Dr. Christina Nassenstein for helpful discussion and Prof. E. Weihe (Philipps-University Marburg) for providing CGRP knockout mice. The authors declare no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shelukhina, I., Paddenberg, R., Kummer, W. et al. Functional expression and axonal transport of α7 nAChRs by peptidergic nociceptors of rat dorsal root ganglion. Brain Struct Funct 220, 1885–1899 (2015). https://doi.org/10.1007/s00429-014-0762-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-014-0762-4