Abstract
The Kölliker–Fuse nucleus (KFN) in dorsolateral pons has been implicated in many physiological functions via its extensive efferent connections. Here, we combine iontophoretic anterograde tracing with posthypoxia c-Fos immunohistology to map KFN axonal terminations among hypoxia-activated/nonactivated brainstem and spinal structures in rats. Using a set of stringent inclusion/exclusion criteria to align visualized axons across multiple coronal brain sections, we were able to unequivocally trace axonal trajectories over a long rostrocaudal distance perpendicular to the coronal plane. Structures that were both richly innervated by KFN axonal projections and immunopositive to c-Fos included KFN (contralateral side), ventrolateral pontine area, areas ventral to rostral compact/subcompact ambiguus nucleus, caudal (lateral) ambiguus nucleus, nucleus retroambiguus, and commissural–medial subdivisions of solitary tract nucleus. The intertrigeminal nucleus, facial and hypoglossal nuclei, retrotrapezoid nucleus, parafacial region and spinal cord segment 5 were also richly innervated by KFN axonal projections but were only weakly (or not) immunopositive to c-Fos. The most striking finding was that some descending axons from KFN sent out branches to innervate multiple (up to seven) pontomedullary target structures including facial nucleus, trigeminal sensory nucleus, and various parts of ambiguus nucleus and its surrounding areas. The extensive axonal fan-out from single KFN neurons to multiple brainstem and spinal cord structures (“one-to-many relationship”) provides anatomical evidence that KFN may coordinate diverse physiological functions including hypoxic and hypercapnic respiratory responses, respiratory pattern generation and motor output, diving reflex, modulation of upper airways patency, coughing and vomiting abdominal expiratory reflex, as well as cardiovascular regulation and cardiorespiratory coupling.










Similar content being viewed by others
Notes
As a historical note, the “Kölliker–Fuse nucleus” as originally described by Kölliker and Fuse (Fuse 1913; Kölliker 1896) in humans and a variety of animal species was subsequently found to actually represent the pedunculopontine tegmental nucleus (Rye et al. 1987). The name “Kölliker–Fuse nucleus” was erroneously given to the dorsolateral pontine structure that bears this name today by Berman in his cat atlas (Berman 1968). Notwithstanding, this mistake has been perpetuated and the “Kölliker–Fuse nucleus” (KFN) in a similar atlas for rat (Paxinos et al. 1999; Paxinos and Watson 1986) is used here to describe the cell group we are studying.
Rudolph Albert von Kölliker coined the term axon in 1896 (Lopez-Munoz and Alamo 2009).
Abbreviations
- 5:
-
Trigeminal motor nucleus
- 7:
-
Facial motor nucleus
- 7n:
-
Facial nerve
- 12:
-
Hypoglossal motor nucleus
- A5:
-
A5 noradrenergic cells
- AMB:
-
Ambiguus nucleus
- rcAMB:
-
Ambiguus nucleus, rostral compact part
- subcAMB:
-
Ambiguus nucleus, subcompact part
- cAMB:
-
Caudal ambiguus nucleus
- BDA:
-
Biotin dextran
- CSN:
-
Carotid sinus nerve
- CVL:
-
Caudal ventrolateral reticular nucleus
- ITN:
-
Intertrigeminal nucleus
- KFN:
-
Kölliker–Fuse nucleus
- LPBN:
-
Lateral parabrachial nucleus
- LPGi:
-
Lateral paragigantocellular reticular nucleus
- LRt:
-
Lateral reticular nucleus
- NRA:
-
Nucleus retroambiguus
- NTS:
-
Nucleus of solitary tract
- vl-NTS:
-
Ventrolateral nucleus of solitary tract
- dl-pons:
-
Dorsolateral pons
- vl-pons:
-
Ventrolateral pons
- P7:
-
Parafacial area
- Pr5VL:
-
Ventrolateral principal sensory trigeminal nucleus
- RTN:
-
Retrotrapezoid nucleus
- RVL:
-
Rostral ventrolateral reticular nucleus
- scp:
-
Superior cerebellum peduncle
- Sp5O:
-
Trigeminal sensory nucleus, oral
- Sp5I:
-
Trigeminal sensory nucleus, interpolar
- ts:
-
Solitary tract
- VLM:
-
Ventrolateral medulla
- VRC:
-
Ventral respiratory neuronal column
References
Alheid GF, Milsom WK, McCrimmon DR (2004) Pontine influences on breathing: an overview. Respir Physiol Neurobiol 143(2–3):105–114
Berman A (1968) The brain stem of the cat: a cytoarchitectonic atlas with stereotaxic coordinates. University of Wisconsin Press, Madison, p 35
Berquin P, Bodineau L, Gros F, Larnicol N (2000) Brainstem and hypothalamic areas involved in respiratory chemoreflexes: a Fos study in adult rats. Brain Res 857(1–2):30–40
Bodineau L, Larnicol N (2001) Brainstem and hypothalamic areas activated by tissue hypoxia: Fos-like immunoreactivity induced by carbon monoxide inhalation in the rat. Neuroscience 108(4):643–653
Bullitt E (1990) Expression of c-fos-like protein as a marker for neuronal activity following noxious stimulation in the rat. J Comp Neurol 296(4):517–530
Burke PG, Abbott SB, McMullan S, Goodchild AK, Pilowsky PM (2010) Somatostatin selectively ablates postinspiratory activity after injection into the Botzinger complex. Neuroscience 167(2):528–539
Chamberlin N (2004) Functional organization of the parabrachial complex and intertrigeminal region in the control of breathing. Respir Physiol Neurobiol 143(2–3):115–125
Chamberlin NL, Saper CB (1992) Topographic organization of cardiovascular responses to electrical and glutamate microstimulation of the parabrachial nucleus in the rat. J Comp Neurol 326(2):245–262
Chamberlin NL, Saper CB (1994) Topographic organization of respiratory responses to glutamate microstimulation of the parabrachial nucleus in the rat. J Neurosci 14(11 Pt 1):6500–6510
Cohen MI (1971) Switching of the respiratory phases and evoked phrenic responses produced by rostral pontine electrical stimulation. J Physiol 217(1):133–158
Cohen MI, Shaw CF (2004) Role in the inspiratory off-switch of vagal inputs to rostral pontine inspiratory-modulated neurons. Respir Physiol Neurobiol 143(2–3):127–140
Cohen MI, Wang SC (1959) Respiratory neuronal activity in pons of cat. J Neurophysiol 22(1):33–50
Coles SK, Dick TE (1996) Neurones in the ventrolateral pons are required for posthypoxic frequency decline in rats. J Physiol 497(Pt 1):79–94
Davis PJ, Nail BS (1984) On the location and size of laryngeal motoneurons in the cat and rabbit. J Comp Neurol 230(1):13–32
Dawid Milner MS, Lara JP, Lopez de Miguel MP, Lopez-Gonzalez MV, Spyer KM, Gonzalez-Baron S (2003) A5 region modulation of the cardiorespiratory responses evoked from parabrachial cell bodies in the anaesthetised rat. Brain Res 982(1):108–118
Dick TE, Bellingham MC, Richter DW (1994) Pontine respiratory neurons in anesthetized cats. Brain Res 636(2):259–269
Dick TE, Baekey DM, Paton JF, Lindsey BG, Morris KF (2009) Cardio-respiratory coupling depends on the pons. Respir Physiol Neurobiol 168(1–2):76–85
Dutschmann M, Herbert H (1996) The Kolliker-Fuse nucleus mediates the trigeminally induced apnoea in the rat. Neuroreport 7(8):1432–1436
Dutschmann M, Herbert H (2006) The Kolliker-Fuse nucleus gates the postinspiratory phase of the respiratory cycle to control inspiratory off-switch and upper airway resistance in rat. Eur J Neurosci 24(4):1071–1084
Dutschmann M, Kron M, Morschel M, Gestreau C (2007) Activation of Orexin B receptors in the pontine Kolliker-Fuse nucleus modulates pre-inspiratory hypoglossal motor activity in rat. Respir Physiol Neurobiol 159(2):232–235
Ellenberger HH, Feldman JL (1990) Brainstem connections of the rostral ventral respiratory group of the rat. Brain Res 513(1):35–42
Erickson JT, Millhorn DE (1994) Hypoxia and electrical stimulation of the carotid sinus nerve induce Fos-like immunoreactivity within catecholaminergic and serotoninergic neurons of the rat brainstem. J Comp Neurol 348(2):161–182
Ezure K (2004) Respiration-related afferents to parabrachial pontine regions. Respir Physiol Neurobiol 143(2–3):167–175
Ezure K, Tanaka I (2006) Distribution and medullary projection of respiratory neurons in the dorsolateral pons of the rat. Neuroscience 141(2):1011–1023
Finley JC, Katz DM (1992) The central organization of carotid body afferent projections to the brainstem of the rat. Brain Res 572(1–2):108–116
Fulwiler CE, Saper CB (1984) Subnuclear organization of the efferent connections of the parabrachial nucleus in the rat. Brain Res 319(3):229–259
Fuse G (1913) Arbeiten aus dem Hirnanatomischen Institut in Zurich. von Monakow C (ed) Wiesbaden: JF Bergmann Verlag. pp. 211–253
Gang S, Mizuguchi A, Aoki M (1991) Axonal projections from the pontine pneumotaxic region to the nucleus raphe magnus in cats. Respir Physiol 85(3):329–339
Gang S, Sato Y, Kohama I, Aoki M (1995) Afferent projections to the Botzinger complex from the upper cervical cord and other respiratory related structures in the brainstem in cats: retrograde WGA-HRP tracing. J Auton Nerv Syst 56(1–2):1–7
Gasparini S, de Luca LAJ, Colombari DS, de Paula PM, Barbosa SP, Menani JV (2009) Adrenergic mechanisms of the Kolliker-Fuse/A7 area on the control of water and sodium intake. Neuroscience 164(2):370–379
Gaytan SP, Calero F, Nunez-Abades PA, Morillo AM, Pasaro R (1997) Pontomedullary efferent projections of the ventral respiratory neuronal subsets of the rat. Brain Res Bull 42(4):323–334
Gerrits PO, Holstege G (1996) Pontine and medullary projections to the nucleus retroambiguus: a wheat germ agglutinin-horseradish peroxidase and autoradiographic tracing study in the cat. J Comp Neurol 373(2):173–185
Gestreau C, Dutschmann M, Obled S, Bianchi AL (2005) Activation of XII motoneurons and premotor neurons during various oropharyngeal behaviors. Respir Physiol Neurobiol 147(2–3):159–176
Gozal D, Xue YD, Simakajornboon N (1999) Hypoxia induces c-Fos protein expression in NMDA but not AMPA glutamate receptor labeled neurons within the nucleus tractus solitarii of the conscious rat. Neurosci Lett 262(2):93–96
Guo ZL, Li P, Longhurst JC (2002) Central pathways in the pons and midbrain involved in cardiac sympathoexcitatory reflexes in cats. Neuroscience 113(2):435–447
Guyenet P (2006) The sympathetic control of blood pressure. Nat Rev Neurosci 7(5):335–346
Haji A, Okazaki M, Yamazaki H, Takeda R (1998) NMDA receptor-mediated inspiratory off-switching in pneumotaxic-disconnected cats. Neurosci Res 32(4):323–331
Herbert H, Moga MM, Saper CB (1990) Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J Comp Neurol 293(4):540–580
Hermes SM, Mitchell JL, Aicher SA (2006) Most neurons in the nucleus tractus solitarii do not send collateral projections to multiple autonomic targets in the rat brain. Exp Neurol 198(2):539–551
Hinrichsen CF, Ryan AT (1981) Localization of laryngeal motoneurons in the rat: morphologic evidence for dual innervation? Exp Neurol 74(2):341–355
Hirooka Y, Polson JW, Potts PD, Dampney RA (1997) Hypoxia-induced Fos expression in neurons projecting to the pressor region in the rostral ventrolateral medulla. Neuroscience 80(4):1209–1224
Histed MH, Bonin V, Reid RC (2009) Direct activation of sparse, distributed populations of cortical neurons by electrical microstimulation. Neuron 63(4):508–522
Hodge CJJ, Apkarian AV, Stevens RT (1986) Inhibition of dorsal-horn cell responses by stimulation of the Kolliker-Fuse nucleus. J Neurosurg 65(6):825–833
Holstege G (1988) Anatomical evidence for a strong ventral parabrachial projection to nucleus raphe magnus and adjacent tegmental field. Brain Res 447(1):154–158
Holstege G, Kuypers HG (1977) Propriobulbar fibre connections to the trigeminal, facial and hypoglossal motor nuclei. I. An anterograde degeneration study in the cat. Brain 100(2):239–264
Hwang JC, Chien CT, St John WM (1988) Characterization of respiratory-related activity of the facial nerve. Respir Physiol 73(2):175–187
Jodkowski JS, Coles SK, Dick TE (1994) A ‘pneumotaxic centre’ in rats. Neurosci Lett 172(1–2):67–72
Jodkowski JS, Coles SK, Dick TE (1997) Prolongation in expiration evoked from ventrolateral pons of adult rats. J Appl Physiol 82(2):377–381
Kalia M (1977) Neuroanatomical organization of the respiratory centers. Federation Proc 36:2405–2411
Kalia MP (1981) Anatomical organization of central respiratory neurons. Annu Rev Physiol 43:105–120
Kölliker A (1896) Handbuch der Gewebelehre des Menschen: Leipzig, W.Englmann. p 874
Krukoff TL, Harris KH, Jhamandas JH (1993) Efferent projections from the parabrachial nucleus demonstrated with the anterograde tracer Phaseolus vulgaris leucoagglutinin. Brain Res Bull 30(1–2):163–172
Kuna ST, Remmers JE (1999) Premotor input to hypoglossal motoneurons from Kolliker-Fuse neurons in decerebrate cats. Respir Physiol 117(2–3):85–95
Lara JP, Parkes MJ, Silva-Carvhalo L, Izzo P, Dawid-Milner MS, Spyer KM (1994) Cardiovascular and respiratory effects of stimulation of cell bodies of the parabrachial nuclei in the anaesthetized rat. J Physiol 477(Pt 2):321–329
Lavezzi AM, Ottaviani G, Rossi L, Matturri L (2004a) Cytoarchitectural organization of the parabrachial/Kolliker-Fuse complex in man. Brain Dev 26(5):316–320
Lavezzi AM, Ottaviani G, Rossi L, Matturri L (2004b) Hypoplasia of the parabrachial/kolliker-fuse complex in perinatal death. Biol Neonate 86(2):92–97
Li YW, Dampney RA (1994) Expression of Fos-like protein in brain following sustained hypertension and hypotension in conscious rabbits. Neuroscience 61(3):613–634
Li Q, Song G (2001) Afferent projection to the retrotrapezoid nucleus from respiratory related structures in the brainstem of rabbit–a retrograde CB-HRP tracing study. Sheng Li Xue Bao 53(5):401–404
Lopez-Munoz F, Alamo C (2009) Historical evolution of the neurotransmission concept. J Neural Transm 116(5):515–533
Lumsden T (1923) Observations on the respiratory centres in the cat. J Physiol London 57:153–160
MacDonald SM, Song G, Poon CS (2007) Nonassociative learning promotes respiratory entrainment to mechanical ventilation. PLoS ONE 2(9):e865
MacDonald SM, Tin C, Song G, Poon CS (2009) Use-dependent learning and memory of the Hering-Breuer inflation reflex in rats. Exp Physiol 94(2):269–278
Mizusawa A, Ogawa H, Kikuchi Y, Hida W, Shirato K (1995) Role of the parabrachial nucleus in ventilatory responses of awake rats. J Physiol 489(Pt 3):877–884
Morgan JI, Cohen DR, Hempstead JL, Curran T (1987) Mapping patterns of c-fos expression in the central nervous system after seizure. Science 237(4811):192–197
Nag S, Mokha SS (2004) Estrogen attenuates antinociception produced by stimulation of Kolliker-Fuse nucleus in the rat. Eur J Neurosci 20(11):3203–3207
Nunez-Abades PA, Morillo AM, Pasaro R (1993) Brainstem connections of the rat ventral respiratory subgroups: afferent projections. J Auton Nerv Syst 42(2):99–118
Onimaru H, Homma I (2003) A novel functional neuron group for respiratory rhythm generation in the ventral medulla. J Neurosci 23(4):1478–1486
Onimaru H, Arata A, Homma I (1987) Localization of respiratory rhythm-generating neurons in the medulla of brainstem-spinal cord preparations from newborn rats. Neurosci Lett 78(2):151–155
Onimaru H, Kumagawa Y, Homma I (2006) Respiration-related rhythmic activity in the rostral medulla of newborn rats. J Neurophysiol 96(1):55–61
Parvizi J, Van Hoesen GW, Damasio A (1998) Severe pathological changes of parabrachial nucleus in Alzheimer’s disease. Neuroreport 9(18):4151–4154
Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates. Academic Press, San Diego
Paxinos G, Carrive P, Wang H, Wang P (1999) Chemoarchitectonic atlas of the rat brainstem. Academic Press, San Diego
Poon C, Song G (2004) Distinct responses of respiratory neurons in rostrolateral pons to vagal stimulation and brief hypoxia. Soc Neurosci Abs 145:4
Potts PD, Polson JW, Hirooka Y, Dampney RA (1997) Effects of sinoaortic denervation on Fos expression in the brain evoked by hypertension and hypotension in conscious rabbits. Neuroscience 77(2):503–520
Rexed B (1954) A cytoarchitectonic atlas of the spinal cord in the cat. J Comp Neurol 100(2):297–379
Rosin DL, Chang DA, Guyenet PG (2006) Afferent and efferent connections of the rat retrotrapezoid nucleus. J Comp Neurol 499(1):64–89
Ryan CM, Bradley TD (2005) Pathogenesis of obstructive sleep apnea. J Appl Physiol 99(6):2440–2450
Rye DB, Saper CB, Lee HJ, Wainer BH (1987) Pedunculopontine tegmental nucleus of the rat: cytoarchitecture, cytochemistry, and some extrapyramidal connections of the mesopontine tegmentum. J Comp Neurol 259(4):483–528
Sagar SM, Sharp FR, Curran T (1988) Expression of c-fos protein in brain: metabolic mapping at the cellular level. Science 240(4857):1328–1331
Saper CB, Loewy AD (1980) Efferent connections of the parabrachial nucleus in the rat. Brain Res 197(2):291–317
Shiba K, Nakazawa K, Ono K, Umezaki T (2007) Multifunctional laryngeal premotor neurons: their activities during breathing, coughing, sneezing, and swallowing. J Neurosci 27(19):5156–5162
Siniaia MS, Young DL, Poon CS (2000) Habituation and desensitization of the Hering-Breuer reflex in rat. J Physiol 523(Pt 2):479–491
Smith JC, Morrison DE, Ellenberger HH, Otto MR, Feldman JL (1989) Brainstem projections to the major respiratory neuron populations in the medulla of the cat. J Comp Neurol 281(1):69–96
Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL (1991) Pre-Botzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science 254(5032):726–729
Smith JC, Abdala AP, Koizumi H, Rybak IA, Paton JF (2007) Spatial and functional architecture of the mammalian brain stem respiratory network: a hierarchy of three oscillatory mechanisms. J Neurophysiol 98(6):3370–3387
Solomon IC, Edelman NH, Neubauer JA (2000) Pre-Botzinger complex functions as a central hypoxia chemosensor for respiration in vivo. J Neurophysiol 83(5):2854–2868
Song G, Poon C (2004) Functional and structural models of pontine modulation of mechanoreceptor and chemoreceptor reflexes. Respir Physiol Neurobiol 143(2–3):281–292
Song G, Poon CS (2009a) Lateral parabrachial nucleus mediates shortening of expiration and increase of inspiratory drive during hypercapnia. Respir Physiol Neurobiol 165(1):9–12
Song G, Poon CS (2009b) Lateral parabrachial nucleus mediates shortening of expiration during hypoxia. Respir Physiol Neurobiol 165(1):1–8
Song G, Yu Y, Poon CS (2006) Cytoarchitecture of pneumotaxic integration of respiratory and nonrespiratory information in the rat. J Neurosci 26(1):300–310
Song G, Tin C, Poon CS (2010) Bilateral lesions of pontine Kolliker-Fuse nuclei provoke apnea instead of apneusis in anesthetized adult rats. Adv Exp Med Biol 669:185–188
Song G, Tin C, Giacometti E, Poon CS (2011a) Habituation without NMDA receptor-dependent desensitization of Hering-Breuer apnea reflex in a Mecp2+/− Mutant Mouse Model of Rett syndrome. Front Integr Neurosci 5:6
Song G, Xu H, Wang H, Macdonald SM, Poon CS (2011b) Hypoxia-excited neurons in NTS send axonal projections to Kolliker-Fuse/parabrachial complex in dorsolateral pons. Neuroscience 175:145–153
St John W (1975) Differing responses to hypercapnia and hypoxia following pneumotaxic center ablation. Respir Physiol 23(1):1–9
Stettner GM, Huppke P, Brendel C, Richter DW, Gartner J, Dutschmann M (2007) Breathing dysfunctions associated with impaired control of postinspiratory activity in Mecp2−/y knockout mice. J Physiol 579(Pt 3):863–876
Strohl K (1985) Respiratory activation of the facial nerve and alar muscles in anaesthetized dogs. J Physiol 363:351–362
Subramanian HH, Holstege G (2009) The nucleus retroambiguus control of respiration. J Neurosci 29(12):3824–3832
Sun QJ, Berkowitz RG, Pilowsky PM (2008) GABA A mediated inhibition and postinspiratory pattern of laryngeal constrictor motoneurons in rat. Respir Physiol Neurobiol 162(1):41–47
Teppema LJ, Veening JG, Kranenburg A, Dahan A, Berkenbosch A, Olievier C (1997) Expression of c-fos in the rat brainstem after exposure to hypoxia and to normoxic and hyperoxic hypercapnia. J Comp Neurol 388(2):169–190
von Euler C, Trippenbach T (1976) Excitability changes of the inspiratory “off-switch” mechanism tested by electrical stimulation in nucleus parabrachialis in the cat. Acta Physiol Scand 97(2):175–188
von Euler C, Marttila I, Remmers JE, Trippenbach T (1976) Effects of lesions in the parabrachial nucleus on the mechanisms for central and reflex termination of inspiration in the cat. Acta Physiol Scand 96(3):324–337
Welker W (1964) Analysis of sniffing of the albino rat. Behavior 22:223–244
Wittmeier S, Song G, Duffin J, Poon CS (2008) Pacemakers handshake synchronization mechanism of mammalian respiratory rhythmogenesis. Proc Natl Acad Sci USA 105(46):18000–18005
Yokota S, Tsumori T, Ono K, Yasui Y (2004) Glutamatergic pathways from the Kolliker-Fuse nucleus to the phrenic nucleus in the rat. Brain Res 995(1):118–130
Young RF, Tronnier V, Rinaldi PC (1992) Chronic stimulation of the Kolliker-Fuse nucleus region for relief of intractable pain in humans. J Neurosurg 76(6):979–985
Yu Y, MacDonald SM, Song G, Poon CS (2006) Multielectrode recording and mapping of neuronal microcircuits in pontine pneumotaxic center. Soc Neurosci Abs 308:305
Zheng Y, Riche D, Rekling JC, Foutz AS, Denavit-Saubie M (1998) Brainstem neurons projecting to the rostral ventral respiratory group (VRG) in the medulla oblongata of the rat revealed by co-application of NMDA and biocytin. Brain Res 782(1–2):113–125
Acknowledgments
This work was supported by National Institutes of Health grants HL067966, HL072849, HL079503, and HL093225.
Author information
Authors and Affiliations
Corresponding author
Appendix: Critique of methodologies
Appendix: Critique of methodologies
Microinjection
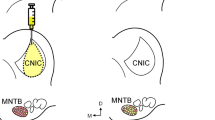
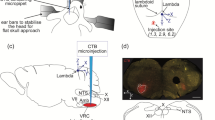
BDA is a proven sensitive and reliable anterograde tracer. With sufficient postinjection survival time (1–2 weeks), even very fine axonal terminals can be sufficiently filled for microscopic observation and long distance tracing. Retrograde labeling has also been reported but only after large injections. In the present study, labeled neurons were observed in areas surrounding the injection site (Fig. 1a) but rarely in other regions. However, BDA is not functionally specific and the KFN is functionally heterogeneous, i.e., besides the well-known pneumotaxic function, KFN is also known to participate in cardiovascular control, anti-nociception, and control of feeding and drinking. To increase the likelihood of labeling neurons that were respiratory-related, we first mapped the KFN with electrical microstimulation for loci that produced respiratory inhibition with the lowest stimulation intensity. Although such electrical microstimulation could activate neurons far from the tip of the stimulating electrode as a result of direct axonal activation (Histed et al. 2009), previous studies have shown that electrical or chemical microstimulation (with glutamate) of neurons in this structure cause similar profound changes in respiratory patterns (Chamberlin and Saper 1992; Cohen 1971; Dutschmann and Herbert 2006; Haji et al. 1998). Thus we believe that the electrical microstimulation-evoked respiratory inhibition in the present study was due to activation of KFN neurons in the vicinity of the electrode tip. Hence, BDA was injected only into such low-threshold regions. With this functionally guided method, we found that the centers of injections in all experimental animals were within the boundaries of KFN and the diffusion of the injectant into neighboring areas was quite limited in most cases. In contrast, failure to identify such low-threshold regions resulted in misplaced injections in three animals. Data from misplaced injections were not included in this report.
In light of the above precautions, we believe that the labeled axons were predominantly from respiratory-related KFN neurons because: (1) the KFN contains the highest density of respiratory-related neurons that send axons to ventrolateral pons and medulla (Ezure and Tanaka 2006; Song et al. 2006); (2) ascending projections from medullary respiratory-related structures predominantly terminate at this structure (Gaytan et al. 1997; Kalia 1977); (3) the highly selective termination of labeled terminals in well-established brainstem respiratory-related structures with c-Fos positive response to hypoxia as demonstrated in this study; (4) BDA injections at control sites neighboring the KFN resulted in very different projection/innervation patterns of the labeled axons. Although nonspecific labeling of axons of other functional modalities (e.g., cardiovascular) from KFN and a minority of axons from neighboring medial and lateral parabrachial nuclei or A7 region (especially for the A7 region) cannot be ruled out especially with pressure injection, useful information about the multi-functional role of the KFN can still be derived from this study.
Axonal tracing
Traditionally, retrograde tracing with a combination of fluorescent tracers of two or three different colors is commonly used to reveal the collateral innervations of a single neuron or axon. However, current fluorescent imaging techniques cannot differentiate the labeling of one neuron by more than three fluorescent tracers. As a result, no more than three innervations can be revealed at a time. In addition, this technique requires 2–3 microinjections at different brain structures, which is rather traumatic to the animal. Because of potential spread of the injectants into neighboring areas, distinct innervations of adjacent target structures cannot be reliably resolved by this method. When more than three structures or when two or more neighboring structures are innervated by branches from such an axon, single-axon anterograde tracing is the only currently available method to reveal these multiple innervations.
On the other hand, long distance anterograde tracing of individual axons over multiple brain sections is generally a challenging task because each section may contain many labeled axons that are difficult to align across consecutive sections. This difficulty has bedeviled early studies using pressure injection, each of which could potentially label hundreds of descending fibers even for a structure with sparse neuronal density such as KFN. To circumvent this difficult, such tracing was performed only in animals with iontophoretic injection in the present study. The number of descending axons labeled after iontophoretic injection varied greatly from five to over one hundred per animal depending on the duration and current intensity of the iontophoresis and the tip diameter of the micropipette. To avoid any ambiguity in the tracing, stem axons that were selected for long distance tracing and reported herein were all relatively large (1.0–2.3 μm diameter) and isolated, i.e., without any neighboring axons of comparable size in each and every section. Such large stem axons typically stood out readily among much smaller axonal branches and were readily identified in coronal sections since they traveled rostrocaudally throughout much of their trajectories from ventrolateral pons to caudal medulla and spinal cord. If the identified axon shifted significantly from its preceding coordinates on the coronal plane or if other axons of comparable size emerged in any section, the tracing was abandoned. These stringent inclusion/exclusion criteria ensured the unequivocal matching of the same stem axon over consecutive brain sections.
In contrast, thinner branches or collaterals arising from such stem axons in any section were typically traced only within the same section to avoid ambiguity; reconstruction of those branches from two or more consecutive sections was performed only when no neighboring fibers with similar diameter and direction of projection were observed in each section. For this reason, some branches could not be traced to their ultimate targets. Fortuitously, unlike the stem axons that projected lengthwise rostrocaudally, such branches or collaterals tended to be much shorter and confined to the coronal planes, with the extensions in sagittal planes rarely spanning beyond three consecutive sections.
With the above precautions, only 1–2 axons of relatively large diameters met our stringent inclusion/exclusion criteria and were traced for various distances in each animal (totally 10 axons in 7 animals). Many of the large-diameter axons could not be traced for a long distance because they followed a more zigzag path that was not always perpendicular to the coronal plane, or simply because there were multiple large-diameter axons in their proximity that confounded the tracing. On the other hand, none of the medium-sized axons was successfully traced for sufficient distance. Nevertheless, branches and collaterals were still observed arising from such axons. In light of these observations, we believe the collateral innervations of multiple target structures might be common (if not universal) among descending axons of both medium and large diameters although many of them might not reach the caudal medulla.
Rights and permissions
About this article
Cite this article
Song, G., Wang, H., Xu, H. et al. Kölliker–Fuse neurons send collateral projections to multiple hypoxia-activated and nonactivated structures in rat brainstem and spinal cord. Brain Struct Funct 217, 835–858 (2012). https://doi.org/10.1007/s00429-012-0384-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-012-0384-7