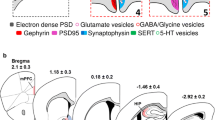
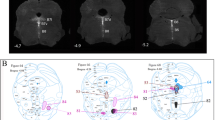
Abstract
It is well established that serotonergic (5-hydroxytryptamine, 5-HT) fibers, mainly originating from the dorsal and median raphe nuclei of the brainstem, distribute throughout the forebrain, most heavily to ‘limbic’ forebrain structures. Few reports have examined the distribution of 5-HT fibers to the thalamus and none to our knowledge using immunoprocedures for the detection of the serotonin transporter (SERT)—a very sensitive marker for 5-HT fibers. Using immunohistochemical methods for SERT, we examined the pattern of distribution of 5-HT fibers to the thalamus in the rat. We show that serotonergic fibers are heavily concentrated in midline, intralaminar and association nuclei of the thalamus, and with the exception of the lateral geniculate complex, weakly distributed to principal nuclei of thalamus. Specifically, we demonstrate that 5-HT fibers are densely concentrated in the anteroventral, anteromedial and interanteromedial nuclei of the anterior thalamus, the paraventricular, rhomboid and reuniens nuclei of the midline thalamus, the central medial and central lateral nuclei of the intralaminar thalamus, the intermediodorsal nucleus, the lateral dorsal nucleus, and the dorsal and ventral lateral geniculate nuclei and intergeniculate leaflet of the LGN complex. Less densely innervated sites include the mediodorsal, paracentral, parafascicular, lateral posterior and submedial nuclei of thalamus. Remaining regions of the thalamus, largely consisting of principal nuclei, contained few 5-HT fibers. This pattern of 5-HT innervation indicates that serotonin/serotonergic fibers mainly affect thalamic nuclei with connections to ‘non-principal’ or limbic regions of the cortex (or forebrain). This suggests that serotonergic fibers to the thalamus may exert a significant influence on affective and cognitive functions, possibly complementing the actions of 5-HT fibers to other parts of the brain involved in emotional and cognitive behaviors.

















Similar content being viewed by others
Abbreviations
- 5-HT:
-
5-Hydroxytryptamine, serotonin
- AD:
-
Anterodorsal nucleus of the thalamus
- AM:
-
Anteromedial nucleus of thalamus
- APN:
-
Anterior pretectal nucleus
- AV:
-
Anteroventral nucleus of thalamus
- CL:
-
Central lateral nucleus of the thalamus
- CM:
-
Central medial nucleus of thalamus
- COM:
-
Commissural nucleus, periaqueductal gray
- cpd:
-
Cerebral peduncle
- DR:
-
Dorsal raphe nucleus
- ec:
-
External capsule
- EC:
-
Entorhinal cortex
- fr:
-
Fasciculus retroflexus
- fx:
-
Fornix
- HD:
-
Head direction
- HF:
-
Hippocampus
- IAD:
-
Interanterodorsal nucleus of thalamus
- IAM:
-
Interanteromedial nucleus of thalamus
- ic:
-
Internal capsule
- IGL:
-
Intergeniculate leaflet
- IL:
-
Intralaminar thalamus
- IMD:
-
Intermediodorsal nucleus of thalamus
- LD:
-
Lateral dorsal nucleus of thalamus
- LGNd:
-
Dorsal lateral geniculate nucleus
- LGNv,m,l:
-
Ventral lateral geniculate nucleus, medial and lateral divisions
- LH:
-
Lateral habenula
- LHy:
-
Lateral hypothalamus
- LPl,m:
-
Lateral posterior nucleus of thalamus, lateral and medial divisions
- MB:
-
Mammillary bodies
- MDc,1,m:
-
Mediodorsal nucleus of thalamus, central, lateral, and medial divisions
- MGN:
-
Medial geniculate nucleus
- MH:
-
Medial habenula
- ml:
-
Medial lemniscus
- MPT:
-
Medial pretectal nucleus
- MR:
-
Median raphe nucleus
- MRF:
-
Mesencephalic reticular formation
- mt:
-
Mammillothalamic tract
- NOT:
-
Nucleus of optic tract
- NPC:
-
Nucleus of posterior commissure
- OP:
-
Olivary pretectal nucleus
- PAG:
-
Periaqueductal gray
- pc:
-
Posterior commissure
- PCN:
-
Paracentral nucleus of thalamus
- PF:
-
Parafascicular nucleus of thalamus
- PFC:
-
Prefrontal cortex
- PH:
-
Posterior hypothalamus
- PO:
-
Posterior nucleus of thalamus
- PR:
-
Peri-reuniens nucleus
- PT:
-
Paratenial nucleus of thalamus
- PVa,p:
-
Paraventricular nucleus of thalamus, anterior and posterior divisions
- RE:
-
Nucleus reuniens of thalamus
- RH:
-
Rhomboid nucleus of thalamus
- RSC:
-
Retrosplenial cortex
- RT:
-
Reticular nucleus of thalamus
- SC:
-
Superior colliculus
- SCN:
-
Suprachiasmatic nucleus
- SERT:
-
Serotonin transporter
- sm:
-
Stria medullaris
- SMT:
-
Submedial nucleus of thalamus
- SPF:
-
Subparafascicular nucleus
- st:
-
Stria terminalis
- VAL:
-
Ventroanterior lateral complex of thalamus
- VB:
-
Ventrobasal complex of the thalamus
- VM:
-
Ventral medial nucleus of thalamus
- ZI:
-
Zona incerta
References
Aggleton JP, Brown MW (1999) Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. Behav Brain Sci 22:425–444
Aggleton JP, Brown MW (2006) Interleaving brain systems for episodic and recognition memory. Trends Cogn Sci 10:455–463
Aggleton JP, Hunt PR, Nagle S, Neave N (1996) The effects of selective lesions within the anterior thalamic nuclei on spatial memory in the rat. Behav Brain Res 81:189–198
Amaral DG, Witter MP (1995) Hippocampal formation. In: Paxinos G (ed) The rat nervous system, 2nd edn. Academic Press, London, pp 443–493
Austin MC, Rhodes JL, Lewis DA (1997) Differential distribution of corticotropin-releasing hormone immunoreactive axons in monoaminergic nuclei of the human brainstem. Neuropsychopharmacology 17:326–341
Azmitia EC, Segal M (1978) An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. J Comp Neurol 179:641–667
Bailey KR, Mair RG (2005) Lesions of specific and nonspecific thalamic nuclei affect prefrontal cortex-dependent aspects of spatial working memory. Behav Neurosci 119:410–419
Bentivoglio M, Balercia G, Kruger L (1991) The specificity of the nonspecific thalamus: the midline nuclei. Prog Brain Res 87:53–80
Beracochea DJ, Jaffard R, Jarrard LE (1989) Effects of anterior or dorsomedial thalamic ibotenic lesions on learning and memory in rats. Behav Neural Biol 51:364–376
Berendse HW, Groenewegen HJ (1990) Organization of the thalamostriatal projections in the rat, with special emphasis on the ventral striatum. J Comp Neurol 299:187–228
Berendse HW, Groenewegen HJ (1991) Restricted cortical termination fields of the midline and intralaminar thalamic nuclei in the rat. Neuroscience 42:73–102
Bezdudnaya T, Keller A (2008) Laterodorsal nucleus of the thalamus: a processor of somatosensory inputs. J Comp Neurol 507:1979–1989
Byatt G, Dalrymple-Alford JC (1996) Both anteromedial and anteroventral thalamic lesions impair radial-maze learning in rats. Behav Neurosci 110:1335–1348
Cassel JC, Jeltsch H (1995) Serotonergic modulation of cholinergic function in the central nervous system: cognitive implications. Neuroscience 69:1–41
Charara A, Parent A (1998) Chemoarchitecture of the primate dorsal raphe nucleus. J Chem Neuroanat 15:111–127
Cools R, Roberts AC, Robbins TW (2008) Serotoninergic regulation of emotional and behavioural control processes. Trends Cogn Sci 12:31–40
Cropper EC, Eisenman JS, Azmitia EC (1984) An immunocytochemical study of the serotonergic innervation of the thalamus of the rat. J Comp Neurol 224:38–50
Datta S, Maclean RR (2007) Neurobiological mechanisms for the regulation of mammalian sleep–wake behavior: reinterpretation of historical evidence and inclusion of contemporary cellular and molecular evidence. Neurosci Biobehav Rev 31:775–824
Day HE, Greenwood BN, Hammack SE, Watkins LR, Fleshner M, Maier SF, Campeau S (2004) Differential expression of 5HT-1A, alpha 1b adrenergic, CRF-R1, and CRF-R2 receptor mRNA in serotonergic, gamma-aminobutyric acidergic, and catecholaminergic cells of the rat dorsal raphe nucleus. J Comp Neurol 474:364–378
Dempsey EW, Morison RS (1942) The production of rhythmically recurrent cortical potentials after localized thalamic stimulation. Am J Physiol 135:293–300
Dempsey EW, Morison RS (1943) The electrical activity of a thalamocortical relay system. Am J Physiol 138:283–298
Dolleman-van der Weel MJ, Morris RG, Witter MP (2009) Neurotoxic lesions of the thalamic reuniens or mediodorsal nucleus in rats affect non-mnemonic aspects of watermaze learning. Brain Struct Funct 213:329–342
Floresco SB, Block AE, Tse MT (2008) Inactivation of the medial prefrontal cortex of the rat impairs strategy set-shifting, but not reversal learning, using a novel, automated procedure. Behav Brain Res 190:85–96
Ghods-Sharifi S, Haluk DM, Floresco SB (2008) Differential effects of inactivation of the orbitofrontal cortex on strategy set-shifting and reversal learning. Neurobiol Learn Mem 89:567–573
Glass JD, DiNardo LA, Ehlen JC (2000) Dorsal raphe nuclear stimulation of SCN serotonin release and circadian phase-resetting. Brain Res 859:224–232
Gonzalo-Ruiz A, Lieberman AR, Sanz-Anquela JM (1995) Organization of serotoninergic projections from the raphé nuclei to the anterior thalamic nuclei in the rat: a combined retrograde tracing and 5-HT immunohistochemical study. J Chem Neuroanat 8:103–115
Graff-Radford NR, Tranel D, Van Hoesen GW, Brandt JP (1990) Diencephalic amnesia. Brain 113:1–25
Groenewegen HJ, Berendse HW (1994) The specificity of the ‘nonspecific’ midline and intralaminar thalamic nuclei. Trends Neurosci 17:52–57
Groenewegen HJ, Witter MP (2004) Thalamus. In: Paxinos G (ed) The rat nervous system, 3rd edn. Academic Press, New York, pp 407–453
Halliday G, Harding A, Paxinos G (2004) The serotonin and tachykinin systems. In: Paxinos G (ed) The rat nervous system, 3rd edn. Academic Press, New York, pp 1205–1256
Harrington ME (1997) The ventral lateral geniculate nucleus and the intergeniculate leaflet: interrelated structures in the visual and circadian systems. Neurosci Biobehav Rev 21:705–727
Horowitz SS, Blanchard JH, Morin LP (2004) Intergeniculate leaflet and ventral lateral geniculate nucleus afferent connections: an anatomical substrate for functional input from the vestibulo-visuomotor system. J Comp Neurol 474:227–245
Hsu DT, Price JL (2009) Paraventricular thalamic nucleus: subcortical connections and innervation by serotonin, orexin, and corticotropin-releasing hormone in macaque monkeys. J Comp Neurol 512:825–848
Hunt PR, Aggleton JP (1998a) An examination of the spatial working memory deficit following neurotoxic medial dorsal thalamic lesions in rats. Behav Brain Res 97:129–141
Hunt PR, Aggleton JP (1998b) Neurotoxic lesions of the dorsomedial thalamus impair the acquisition but not the performance of delayed matching to place by rats: a deficit in shifting response rules. J Neurosci 18:10045–10052
Jacobs BL, Azmitia EC (1992) Structure and function of the brain serotonin system. Physiol Rev 72:165–229
Jones EG (2007) The thalamus, 2nd edn. Cambridge University Press, Cambridge, UK
Kirouac GJ, Parsons MP, Li S (2005) Orexin (hypocretin) innervation of the paraventricular nucleus of the thalamus. Brain Res 1059:179–188
Kirouac GJ, Parsons MP, Li S (2006) Innervation of the paraventricular nucleus of the thalamus from cocaine- and amphetamine-regulated transcript (CART) containing neurons of the hypothalamus. J Comp Neurol 497:155–165
Kocsis B, Vertes RP (1992) Dorsal raphe neurons: synchronous discharge with the theta rhythm of the hippocampus in the freely behaving rat. J Neurophysiol 68:1463–1467
Kocsis B, Vertes RP (1996) Midbrain raphe cell firing and hippocampal theta rhythm urethane-anaesthetized rats. Neuroreport 7:2867–2872
Kocsis B, Varga V, Dahan L, Sik A (2006) Serotonergic neuron diversity: identification of raphe neurons with discharges time-locked to the hippocampal theta rhythm. Proc Natl Acad Sci USA 2103:1059–1064
Kolmac C, Mitrofanis J (2000) Organization of brain stem afferents to the ventral lateral geniculate nucleus of rats. Vis Neurosci 17:313–318
Kolmac CI, Power BD, Mitrofanis J (2000) Dorsal thalamic connections of the ventral lateral geniculate nucleus of rats. J Neurocytol 29:31–41
Krout KE, Belzer RE, Loewy AD (2002) Brainstem projections to midline and intralaminar thalamic nuclei of the rat. J Comp Neurol 448:53–101
Lacroix L, White I, Feldon J (2002) Effect of excitotoxic lesions of rat medial prefrontal cortex on spatial memory. Behav Brain Res 133:69–81
Lavoie B, Parent A (1991) Serotoninergic innervation of the thalamus in the primate: an immunohistochemical study. J Comp Neurol 312:1–18
Li S, Kirouac GJ (2008) Projections from the paraventricular nucleus of the thalamus to the forebrain, with special emphasis on the extended amygdala. J Comp Neurol 506:263–287
Lowry CA, Evans AK, Gasser PJ, Hale MW, Staub DR, Shekhar A (2008a) Topographic organization and chemoarchitecture of the dorsal raphe nucleus and the median raphe nucleus. In: Monti JM, Pandi-Perumal SR, Jacobs BL, Nutt DJ (eds) Serotonin and sleep: molecular, functional and clinical aspects. Birkhäuser, Basel, Switzerland, pp 25–67
Lowry CA, Hale MW, Evans AK, Heerkens J, Staub DR, Gasser PJ, Shekhar A (2008b) Serotonergic systems, anxiety, and affective disorder: focus on the dorsomedial part of the dorsal raphe nucleus. Ann N Y Acad Sci 1148:86–94
McAlonan GM, Robbins TW, Everitt BJ (1993) Effects of medial dorsal thalamic and ventral pallidal lesions on the acquisition of a conditioned place preference: further evidence for the involvement of the ventral striatopallidal system in reward-related processes. Neuroscience 52:605–620
McKenna JT, Vertes RP (2004) Afferent projections to nucleus reuniens of the thalamus. J Comp Neurol 480:115–142
Melander T, Hökfelt T, Rökaeus A, Cuello AC, Oertel WH, Verhofstad A, Goldstein M (1986) Coexistence of galanin-like immunoreactivity with catecholamines, 5-hydroxytryptamine, GABA and neuropeptides in the rat CNS. J Neurosci 6:3640–3654
Meyer-Bernstein EL, Morin LP (1996) Differential serotonergic innervation of the suprachiasmatic nucleus and the intergeniculate leaflet and its role in circadian rhythm modulation. J Neurosci 16:2097–2111
Mitchell AS, Dalrymple-Alford JC (2005) Dissociable memory effects after medial thalamus lesions in the rat. Eur J Neurosci 22:973–985
Mizumori SJ, Williams JD (1993) Directionally selective mnemonic properties of neurons in the lateral dorsal nucleus of the thalamus of rats. J Neurosci 13:4015–4028
Mizumori SJ, Miya DY, Ward KE (1994) Reversible inactivation of the lateral dorsal thalamus disrupts hippocampal place representation and impairs spatial learning. Brain Res 644:168–174
Moga MM, Weis RP, Moore RY (1995) Efferent projections of the paraventricular thalamic nucleus in the rat. J Comp Neurol 359:221–238
Moore RY, Halaris AE, Jones BE (1978) Serotonin neurons of the midbrain raphe: ascending projections. J Comp Neurol 180:417–438
Moore RY, Speh JC, Card JP (1995) The retinohypothalamic tract originates from a distinct subset of retinal ganglion cells. J Comp Neurol 352:351–366
Moore RY, Weis R, Moga MM (2000) Efferent projections of the intergeniculate leaflet and the ventral lateral geniculate nucleus in the rat. J Comp Neurol 420:398–418
Morin LP, Blanchard JH (1999) Forebrain connections of the hamster intergeniculate leaflet: comparison with those of ventral lateral geniculate nucleus and retina. Vis Neurosci 16:1037–1054
Morin LP, Meyer-Bernstein EL (1999) The ascending serotonergic system in the hamster: comparison with projections of the dorsal and median raphe nuclei. Neuroscience 91:81–105
Morin LP, Pace L (2002) The intergeniculate leaflet, but not the visual midbrain, mediates hamster circadian rhythm response to constant light. J Biol Rhythms 17:217–226
Morison RS, Dempsey EW (1942) A study of thalamocortical relations. Am J Physiol 135:281–292
Nielsen K, Brask D, Knudsen GM, Aznar S (2006) Immunodetection of the serotonin transporter protein is a more valid marker for serotonergic fibers than serotonin. Synapse 59:270–276
Novak CM, Harris JA, Smale L, Nunez AA (2000a) Suprachiasmatic nucleus projections to the paraventricular thalamic nucleus in nocturnal rats (Rattus norvegicus) and diurnal nile grass rats (Arviacanthis niloticus). Brain Res 874:147–157
Novak CM, Smale L, Nunez AA (2000b) Rhythms in Fos expression in brain areas related to the sleep–wake cycle in the diurnal Arvicanthis niloticus. Am J Physiol Regul Integr Comp Physiol 278:R1267–R1274
Otake K, Ruggiero DA (1995) Monoamines and nitric oxide are employed by afferents engaged in midline thalamic regulation. J Neurosci 15:1891–1911
Papez JW (1937) A proposed mechanism for emotion. Arch Neurol Psychiatry 38:725–743
Peng ZC, Bentivoglio M (2004) The thalamic paraventricular nucleus relays information from the suprachiasmatic nucleus to the amygdala: a combined anterograde and retrograde tracing study in the rat at the light and electron microscopic levels. J Neurocytol 33:101–116
Peng ZC, Grassi-Zucconi G, Bentivoglio M (1995) Fos-related protein expression in the midline paraventricular nucleus of the rat thalamus: basal oscillation and relationship with limbic efferents. Exp Brain Res 104:21–29
Peschanski M, Besson JM (1984) Diencephalic connections of the raphé nuclei of the rat brainstem: an anatomical study with reference to the somatosensory system. J Comp Neurol 224:509–534
Price JL (1995) Thalamus. In: Paxinos G (ed) The rat nervous system, 2nd edn. Academic Press, New York, pp 629–648
Risold PY, Thompson RH, Swanson LW (1997) The structural organization of connections between hypothalamus and cerebral cortex. Brain Res Rev 24:197–254
Saper CB, Cano G, Scammell TE (2005a) Homeostatic, circadian, and emotional regulation of sleep. J Comp Neurol 493:92–98
Saper CB, Scammell TE, Lu J (2005b) Hypothalamic regulation of sleep and circadian rhythms. Nature 437:1257–1263
Shibata H, Naito J (2007) Organization of anterior cingulate and frontal cortical projections to the anterior and laterodorsal thalamic nuclei in the rat. Brain Res 1059:93–103
Steinbusch HW (1981) Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience 6:557–618
Su HS, Bentivoglio M (1990) Thalamic midline cell populations projecting to the nucleus accumbens, amygdala, and hippocampus in the rat. J Comp Neurol 297:582–593
Sur C, Betz H, Schloss P (1996) Immunocytochemical detection of the serotonin transporter in rat brain. Neuroscience 73:217–231
Swanson LW (2003) Brain maps: structure of the rat brain. Elsevier, New York
Taube JS (1998) Head direction cells and the neurophysiological basis for a sense of direction. Prog Neurobiol 55:225–256
Thompson SM, Robertson RT (1987) Organization of subcortical pathways for sensory projections to the limbic cortex. II. Afferent projections to the thalamic lateral dorsal nucleus in the rat. J Comp Neurol 265:189–202
Trulson ME, Cannon MS, Raese JD (1985) Identification of dopamine-containing cell bodies in the dorsal and median raphe nuclei of the rat brain using tyrosine hydroxylase immunochemistry. Brain Res Bull 15:229–234
Van der Werf YD, Witter MP, Uylings HB, Jolles J (2000) Neuropsychology of infarctions in the thalamus: a review. Neuropsychologia 38:613–627
Van der Werf YD, Witter MP, Groenewegen HJ (2002) The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res Rev 39:107–140
Van der Werf YD, Jolles J, Witter MP, Uylings HB (2003a) Contributions of thalamic nuclei to declarative memory functioning. Cortex 39:1047–1062
Van der Werf YD, Scheltens P, Lindeboom J, Witter MP, Uylings HB, Jolles J (2003b) Deficits of memory, executive functioning and attention following infarction in the thalamus; a study of 22 cases with localised lesions. Neuropsychologia 41:1330–1344
van Groen T, Wyss JM (1992) Projections from the laterodorsal nucleus of the thalamus to the limbic and visual cortices in the rat. J Comp Neurol 324:427–448
van Groen T, Kadish I, Wyss JM (2002a) Role of the anterodorsal and anteroventral nuclei of the thalamus in spatial memory in the rat. Behav Brain Res 132:19–28
van Groen T, Kadish I, Wyss JM (2002b) The role of the laterodorsal nucleus of the thalamus in spatial learning and memory in the rat. Behav Brain Res 136:329–337
Vann SD (2010) Re-evaluating the role of the mammillary bodies in memory. Neuropsychologia (in press)
Vann SD, Aggleton JP (2004) Deficits of memory, executive functioning and attention following infarction in the thalamus; a study of 22 cases with localised lesions. Nat Rev Neurosci 5:35–44
Vertes RP (1991) A PHA-L analysis of ascending projections of the dorsal raphe nucleus in the rat. J Comp Neurol 313:643–668
Vertes RP (2006) Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience 142:1–20
Vertes RP (2010) Serotonergic regulation of rhythmical activity of the brain, concentrating on the hippocampus. In: Muller C, Jacobs B (eds) Handbook of the behavioral neurobiology of serotonin. Academic, London, pp 277–292
Vertes RP, Crane AM (1997) Distribution, quantification, and morphological characteristics of serotonin-immunoreactive cells of the supralemniscal nucleus (B9) and pontomesencephalic reticular formation in the rat. J Comp Neurol 378:411–424
Vertes RP, Hoover WB (2008) Projections of the paraventricular and paratenial nuclei of the dorsal midline thalamus in the rat. J Comp Neurol 508:212–237
Vertes RP, Linley SB (2007) Comparisons of projections of the dorsal and median raphe nuclei, with some functional considerations. In: Takai K (ed) The interdisciplinary conference on tryptophan and related substances: chemistry, biology, and medicine. International Congress Series, 1304. Elsevier, Oxford, pp 98–120
Vertes RP, Linley SB (2008) Efferent and afferent connections of the dorsal and median raphe nuclei in the rat. In: Monti JM, Pandi-Perumal SR, Jacobs BL, Nutt DJ (eds) Serotonin and sleep: molecular, functional and clinical aspects. Birkhäuser, Basel, Switzerland, pp 69–102
Vertes RP, Martin GF (1988) Autoradiographic analysis of ascending projections from the pontine and mesencephalic reticular formation and the median raphe nucleus in the rat. J Comp Neurol 275:511–541
Vertes RP, Fortin WJ, Crane AM (1999) Projections of the median raphe nucleus in the rat. J Comp Neurol 407:555–582
Vertes RP, Albo Z, Viana Di Prisco G (2001) Theta-rhythmically firing neurons in the anterior thalamus: implications for mnemonic functions of Papez’s circuit. Neuroscience 104:619–625
Vertes RP, Hoover WB, Viana Di Prisco G (2004) Theta rhythm of the hippocampus: subcortical control and functional significance. Behav Cogn Neurosci Rev 3:173–200
Vertes RP, Hoover WB, Do Valle AC, Sherman A, Rodriguez JJ (2006) Efferent projections of reuniens and rhomboid nuclei of the thalamus in the rat. J Comp Neurol 499:768–796
Villar MJ, Vitale ML, Hökfelt T, Verhofstad AA (1988) Dorsal raphe serotoninergic branching neurons projecting both to the lateral geniculate body and superior colliculus: a combined retrograde tracing-immunohistochemical study in the rat. J Comp Neurol 277:126–140
von Cramon DY, Hebel N, Schuri U (1985) A contribution to the anatomical basis of thalamic amnesia. Brain 108:993–1008
Vrang N, Mrosovsky N, Mikkelsen JD (2003) Afferent projections to the hamster intergeniculate leaflet demonstrated by retrograde and anterograde tracing. Brain Res Bull 59:267–288
Warburton EC, Baird AL, Aggleton JP (1997) Assessing the magnitude of the allocentric spatial deficit associated with complete loss of the anterior thalamic nuclei in rats. Behav Brain Res 87:223–232
Waselus M, Van Bockstaele EJ (2007) Co-localization of corticotropin-releasing factor and vesicular glutamate transporters within axon terminals of the rat dorsal raphe nucleus. Brain Res 1174:53–65
Waterhouse BD, Border B, Wahl L, Mihailoff GA (1993) Topographic organization of rat locus coeruleus and dorsal raphe nuclei: distribution of cells projecting to visual system structures. J Comp Neurol 336:345–361
Wilton LA, Baird AL, Muir JL, Honey RC, Aggleton JP (2001) Loss of the thalamic nuclei for “head direction” impairs performance on spatial memory tasks in rats. Behav Neurosci 115:861–869
Wolff M, Loukavenko EA, Will BE, Dalrymple-Alford JC (2008) The extended hippocampal-diencephalic memory system: enriched housing promotes recovery of the flexible use of spatial representations after anterior thalamic lesions. Hippocampus 18:996–1007
Wouterlood FG, Saldana E, Witter MP (1990) Projection from the nucleus reuniens thalami to the hippocampal region: light and electron microscopic tracing study in the rat with the anterograde tracer Phaseolus vulgaris-leucoagglutinin. J Comp Neurol 296:179–203
Zhou FC, Xu Y, Bledsoe S, Lin R, Kelley MR (1996) Serotonin transporter antibodies: production, characterization, and localization in the brain. Mol Brain Res 43:267–278
Acknowledgments
This research was supported by National Science Foundation grant IOS 0820639 to RPV.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vertes, R.P., Linley, S.B. & Hoover, W.B. Pattern of distribution of serotonergic fibers to the thalamus of the rat. Brain Struct Funct 215, 1–28 (2010). https://doi.org/10.1007/s00429-010-0249-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-010-0249-x