Abstract
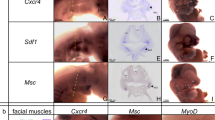
The scapula is a component of the shoulder girdle. Its structure has changed greatly during evolution. For example, in humans it is a large quite flat triangular bone whereas in chicks it is a long blade like structure. In this review we describe the mechanisms that control the formation of the scapula. To assimilate our understanding regarding the development of the scapula blade we start by addressing the issue concerning the origin of the scapula. Experiments using somite extirpation, chick-quail cell marking system and genetic cell labelling techniques in a variety of species have suggested that the scapula had its origin in the somites. For example we have shown in the chick that the scapula blade originates from the somite, while the cranial part, which articulates with the upper limb, is derived from the somatopleure of the forelimb field. In the second and third part of the review we discuss the compartmental origin of this bone and the signalling molecules that control the scapula development. It is very interesting that the scapula blade originates from the dorsal compartment, dermomyotome, which has been previously been associated as a source of muscle and dermis, but not of cartilage. Thus, the development of the scapula blade can be considered a case of dermomyotomal chondrogenesis. Our results show that the dermomyotomal chondrogenesis differ from the sclerotomal chondrogenesis. Firstly, the scapula precursors are located in the hypaxial domain of the dermomyotome, from which the hypaxial muscles are derived. The fate of the scapula precursors, like the hypaxial muscle, is controlled by ectoderm-derived signals and BMPs from the lateral plate mesoderm. Ectoderm ablation and inhibition of BMP activity interfers the scapula-specific Pax1 expression and scapula blade formation. However, only somite cells in the cervicothoracic transition region appear to be committed to form scapula. This indicates that the intrinsic segment specific information determines the scapula forming competence of the somite cells. Taken together, we conclude that the scapula forming cells located within the hypaxial somitic domain require BMP signals derived from the somatopleure and as yet unidentified signals from ectoderm for activation of their coded intrinsic segment specific chondrogenic programme. In the last part we discuss the new data that provides evidence that neural crest contributes for the development of the scapula.



Similar content being viewed by others
References
Balling R, Deutsch U, Gruss P (1988) Undulated, a mutation affecting the development of the mouse skeleton, has a point mutation in the paired box of Pax 1. Cell 55:531–535
Burke AC (1991a) Proximal elements in the vertebrate limb: evolutionary and developmental origin of the pectorial girdle. Plenum, New York
Burke AC (1991b) The development and evolution of the turtle body plan: inferring intrinsic aspects of the evolutionary process from experimental embryology. Am Zool 31:616–627
Chevallier A, Kieny M, Mauger A (1977) Limb–somite relationship: origin of the limb musculature. J Embryol Exp Morphol 41:245–258
Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA (1996) Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature 383:407–413
Christ B, Ordahl CP (1995) Early stages of chick somite development. Anat Embryol (Berl) 191:381–396
Christ B, Jacob HJ, Jacob M (1974) Origin of wing musculature. Experimental studies on quail and chick embryos. Experientia 30:1446–1449
Christ B, Huang R, Wilting J (2000) The development of the avian vertebral column. Anat Embryol (Berl) 202:179–194
Dietrich S (1999) Regulation of hypaxial muscle development. Cell Tissue Res 296:175–182
Dietrich S, Schubert FR, Lumsden A (1997) Control of dorsoventral pattern in the chick paraxial mesoderm. Development 124:3895–3908
Dietrich S, Schubert FR, Healy C, Sharpe PT, Lumsden A (1998) Specification of the hypaxial musculature. Development 125:2235–2249
Ehehalt F, Wang B, Christ B, Patel K, Huang R (2004) Intrinsic cartilage-forming potential of dermomyotomal cells requires ectodermal signals for the development of the scapula blade. Anat Embryol (Berl) 208:431–437
Fomenou MD, Scaal M, Stockdale FE, Christ B, Huang R (2005) Cells of all somitic compartments are determined with respect to segmental identity. Dev Dyn 233:1386–1393
Geetha-Loganathan P, Nimmagadda S, Prols F, Patel K, Scaal M, Huang R, Christ B (2005) Ectodermal Wnt-6 promotes Myf5–dependent avian limb myogenesis. Dev Biol 288:221–233
Geetha-Loganathan P, Nimmagadda S, Huang R, Scaal M, Christ B (2006a) Role of Wnt-6 in limb myogenesis. Anat Embryol (Berl) 211:183–188
Geetha-Loganathan P, Nimmagadda S, Huang R, Christ B, Scaal M (2006b) Regulation of ectodermal Wnt6 expression by the neural tube is transduced by dermomyotomal Wnt11: a mechanism of dermomyotomal lip sustainment. Development 133:2897–2904
Huang R, Zhi Q, Schmidt C, Wilting J, Brand-Saberi B, Christ B (2000a) Sclerotomal origin of the ribs. Development 127:527–532
Huang R, Zhi Q, Patel K, Wilting J, Christ B (2000b) Dual origin and segmental organisation of the avian scapula. Development 127:3789–3794
Hui CC, Joyner AL (1993) A mouse model of greig cephalopolysyndactyly syndrome: the extra-toesJ mutation contains an intragenic deletion of the Gli3 gene. Nat Genet 3:241–246
Kessel M, Gruss P (1991) Homeotic transformations of murine vertebrae and concomitant alteration of Hox codes induced by retinoic acid. Cell 67:89–104
Kuijper S, Beverdam A, Kroon C, Brouwer A, Candille S, Barsh G, Meijlink F (2005) Genetics of shoulder girdle formation: roles of Tbx15 and aristaless-like genes. Development 132:1601–1610
Matsuoka T, Ahlberg PE, Kessaris N, Iannarelli P, Dennehy U, Richardson WD, McMahon AP, Koentges G (2005) Neural crest origins of the neck and shoulder. Nature 436:347–355
Moeller C, Swindell EC, Kispert A, Eichele G (2003) Carboxypeptidase Z (CPZ) modulates Wnt signaling and regulates the development of skeletal elements in the chicken. Development 130:5103–5111
Otto A, Schmidt C, Patel K (2006) Pax3 and Pax7 expression and regulation in the avian embryo. Anat Embryol (Berl) 211:293–310
Pourquie O, Fan CM, Coltey M, Hirsinger E, Watanabe Y, Breant C, Francis-West P, Brickell P, Tessier-Lavigne M, Le Douarin NM (1996) Lateral and axial signals involved in avian somite patterning: a role for BMP4. Cell 84:461–471
Prols F, Ehehalt F, Rodriguez-Niedenfuhr M, He L, Huang R, Christ B (2004) The role of Emx2 during scapula formation. Dev Biol 275:315–324
Schmidt C, Christ B, Patel K, Brand-Saberi B (1998) Experimental induction of BMP-4 expression leads to apoptosis in the paraxial and lateral plate mesoderm. Dev Biol 202:253–263
Schmidt C, Christ B, Maden M, Brand-Saberi B, Patel K (2001) Regulation of Epha4 expression in paraxial and lateral plate mesoderm by ectoderm-derived signals. Dev Dyn 220:377–386
Schmidt C, Stoeckelhuber M, McKinnell I, Putz R, Christ B, Patel K (2004) Wnt 6 regulates the epithelialisation process of the segmental plate mesoderm leading to somite formation. Dev Biol 271:198–209
Schoenwolf GC, Garcia-Martinez V, Dias MS (1992) Mesoderm movement and fate during avian gastrulation and neurulation. Dev Dyn 193:235–248
Selleck MA, Stern CD (1991) Fate mapping and cell lineage analysis of Hensen’s node in the chick embryo. Development 112:15–26
Singh MK, Petry M, Haenig B, Lescher B, Leitges M, Kispert A (2005) The T-box transcription factor Tbx15 is required for skeletal development. Mech Dev 122:131–144
Sosic D, Brand-Saberi B, Schmidt C, Christ B, Olson EN (1997) Regulation of paraxis expression and somite formation by ectoderm- and neural tube-derived signals. Dev Biol 185:29–43
Teillet M, Watanabe Y, Jeffs P, Duprez D, Lapointe F, Le Douarin NM (1998) Sonic hedgehog is required for survival of both myogenic and chondrogenic somitic lineages. Development 125:2019–2030
Wang B, He L, Ehehalt F, Geetha-Loganathan P, Nimmagadda S, Christ B, Scaal M, Huang R (2005) The formation of the avian scapula blade takes place in the hypaxial domain of the somites and requires somatopleure-derived BMP signals. Dev Biol 287:11–18
Acknowledgments
KP wishes to thank the Wellcome Trust (grant 077750) for providing funds enabling this work. This work was supported by grants of the Deutsche Forschungsgemeinschaft (Hu729/2) to RH. We apologise to those authors whose work has not been cited in full. This is due to constraints in manuscript length.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, R., Christ, B. & Patel, K. Regulation of scapula development. Brain Struct Funct 211 (Suppl 1), 65–71 (2006). https://doi.org/10.1007/s00429-006-0126-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-006-0126-9