Abstract
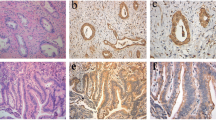
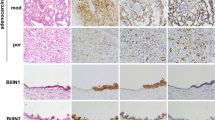
The claudin family members are the functional components of tight junctions. Expression and localization of claudins vary among organs and tumor types. In this study, we examined expression and localization of tight junction proteins (TJP) in human liver tumors, to estimate their usefulness as differential diagnostic markers. The materials used for immunohistochemical analysis were 47 liver tumor specimens including 29 cases of hepatocellular carcinoma (HCC), 15 cases of cholangiocarcinoma (CC), 3 cases of combined HCC and CC (CHC), and 3 cases of cholangiolocellular carcinoma (CoCC). Samples were examined using semiquantitative and statistical analysis of immunoreactivity. In HCC, claudin-1, occludin, tricellulin, and JAM-A were expressed on the cell membrane as well as in hepatocytes. In CC, claudins-1, -4, and -7, tricellulin, and JAM-A were expressed on the cell membrane and occludin was predominantly expressed in the apicalmost areas of the cell membrane. Significant differences in the immunohistochemical scores of claudin-4 and claudin-7 were observed when comparing HCC and CC. CHC was positive for all of the TJPs examined in this study. The expression pattern of CoCC was found to be similar to that of CC. There were differences in the distribution of intensity scores of claudins-4 and -7 and occludin between CoCC and HCC. In addition, CHC was positive for Glypican-3 and CK-19. CoCC was positive for only CK-19. The results suggest that claudins-4 and -7 might be valuable markers for distinguishing HCC and CC and that CoCC might arise from hepatic ductal cells.





Similar content being viewed by others
References
Anderson JM, Cereijido M (2001) Tight junctions, 2nd edn. CRC Press, Boca Raton, pp 1–18
Tsukita S, Furuse M, Itoh M (2001) Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol 2:285–293
Kojima T, Murata M, Yamamoto T, Lan M, Imamura M, Son S, Takano K, Yamaguchi H, Ito T, Tanaka S, Chiba H, Hirata K, Sawada N (2009) Tight junction proteins and signal transduction pathways in hepatocytes. Histol Histopathol 24:1463–1472
Kojima T, Sawada N, Yamaguchi H, Fort AG, Spray DC (2009) Gap and tight junctions in liver: composition, regulation, and function. In: Arias IM et al (eds) The liver: biology and pathobiology, 5th edn. Lippincott Williams & Wilkins, Philadelphia, pp 201–220
Kojima T, Sawada N (2011) Expression and function of claudins in hepatocytes. Methods Mol Biol 762:233–244
Sawada N, Murata M, Kikuchi K, Osanai M, Tobioka H, Kojima T, Chiba H (2003) Tight junctions and human diseases. Med Electron Microsc 36:147–156
Chiba H, Osanai M, Murata M, Kojima T, Sawada N (2008) Transmembrane proteins of tight junctions. Biochim Biophys Acta 1778:588–600
Jakab C, Kiss A, Schaff Z, Szabó Z, Rusvai M, Gálfi P, Szabára A, Sterczer A, Kulka J (2010) Claudin-7 protein differentiates canine cholangiocarcinoma from hepatocellular carcinoma. Histol Histopathol 25:857–864
Tsujiwaki M, Murata M, Takasawa A, Hiratsuka Y, Fukuda R, Sugimoto K, Ono Y, Nojima M, Tanaka S, Hirata K, Kojima T, Sawada N (2015) Aberrant expression of claudin-4 and −7 in hepatocytes in the cirrhotic human liver. Med Mol Morphol 48:33–34
González-Mariscal L, Tapia R, Chamorro D (2008) Crosstalk of tight junction components with signaling pathways. Biochim Biophys Acta 1778:729–756
Sawada N (2013) Tight junction-related human diseases. Pathol Int 63:1–12
Morin PJ (2005) Claudin proteins in human cancer: promising new targets for diagnosis and therapy. Cancer Res 65:9603–9606
Kominsky SL (2006) Claudins: emerging targets for cancer therapy. Expert Rev Mol Med 8:1–11
Oliveira SS, Morgado-Díaz JA (2007) Claudins: multifunctional players in epithelial tight junctions and their role in cancer. Cell Mol Life Sci 64:17–28
Maeda T, Murata M, Chiba H, Takasawa A, Tanaka S, Kojima T, Masumori N, Tsukamoto T, Sawada N (2012) Claudin-4-targeted therapy using Clostridium perfringens enterotoxin for prostate cancer. Prostate 72:351–360
Soini Y, Takasawa A, Eskelinen M, Juvonen P, Kärjä V, Hasegawa T, Murata M, Tanaka S, Kojima T, Sawada N (2012) Expression of claudins 7 and 18 in pancreatic ductal adenocarcinoma: association with features of differentiation. J Clin Pathol 65:431–436
Keira Y, Takasawa A, Murata M, Nojima M, Takasawa K, Ogino J, Higashiura Y, Sasaki A, Kimura Y, Mizuguchi T, Tanaka S, Hirata K, Sawada N, Hasegawa T (2015) An immunohistochemical marker panel including claudin-18, maspin, and p53 improves diagnostic accuracy of bile duct neoplasms in surgical and presurgical biopsy specimens. Virchows Arch 466:265–277
Offner S, Hekele A, Teichmann U, Weinberger S, Gross S, Kufer P, Itin C, Baeuerle PA, Kohleisen B (2005) Epithelial tight junction proteins as potential antibody targets for pancarcinoma therapy. Cancer Immunol Immunother 54:431–445
London WT, McGlynn KA (2006) Liver cancer. In: Schottenfeld D, Fraumeni J Jr (eds) Cancer epidemiology and prevention, 3rd edn. Oxford University Press, New York, pp 763–786
Lindsey AT, Freddie B, Rebecca LS, Jacques F, Joannie LT, Ahmedin J (2012) Global cancer statistics, 2012. Cancer J Clin 65:87–108
Roskams T (2006) Liver stem cells and their implication in hepatocellular and cholangiocarcinoma. Oncogene 25:3818–3822
Allen RA, Lisa JR (1949) Combined liver cell and bile duct carcinoma. Am J Pathol 25:647–655
Fowler KJ, Sheybani A, Parker RA 3rd, Doherty S, M Brunt E, Chapman WC, Menias CO (2013) Combined hepatocellular and cholangiocarcinoma (biphenotypic) tumors: imaging features and diagnostic accuracy of contrast-enhanced CT and MRI. Am J Roentgenol 201:332–339
Libbrecht L (2006) Hepatic progenitor cells in human liver tumor development. World J Gastroenterol 12:6261–6265
Steiner PE, Higginson J (1959) Cholangiolocellular carcinoma of the liver. Cancer 12:753–759
Shiota K, Taguchi J, Nakashima O, Nakashima M, Kojiro M (2001) Clinicopathologic study on cholangiolocellular carcinoma. Oncol Rep 8:263–268
Kozaka K, Sasaki M, Fujii T, Harada K, Zen Y, Sato Y, Sawada S, Minato H, Matsui O, Nakanuma Y (2007) A subgroup of intrahepatic cholangiocarcinoma with an infiltrating replacement growth pattern and a resemblance to reactive proliferating bile ductules: bile ductular carcinoma. Histopathology 51:390–400
Komuta M, Spee B, Vander Borght S, De Vos R, Verslype C, Aerts R, Yano H, Suzuki T, Matsuda M, Fujii H, Desmet VJ, Kojiro M, Roskams T (2008) Clinicopathological study on cholangiolocellular carcinoma suggesting hepatic progenitor cell origin. Hepatology 47:1544–1556
Liver Cancer Study Group of Japan (2009) The general rules for the clinical and pathological study of primary liver cancer. 5th ed. Revised version. Kanehara, Tokyo
Theise ND, Nakashima O, Park YN, Nakanuma Y (2010) Combined hepatocellular-cholangiocarcinoma. In: Bosman FT, Carneiro F, Hruban RH, Theise ND (eds) World health organization classification of tumors. WHO Classification of Tumors of the Digestive System. International Agency for Research on Cancer, Lyon, pp 225–227
Iwahashi S, Utsunomiya T, Shimada M, Saito Y, Morine Y, Imura S, Ikemoto T, Mori H, Hanaoka J, Bando Y (2013) High expression of cancer stem cell markers in cholangiolocellular carcinoma. Surg Today 43:654–660
Kondo F, Fukusato T (2015) Pathogenesis of cholangiolocellular carcinoma: possibility of an interlobular duct origin. Intern Med 54:1685–1694
Ryu HS, Lee K, Shin E, Kim SH, Jing J, Jung HY, Lee H, Jang JJ (2012) Comparative analysis of immunohistochemical markers for differential diagnosis of hepatocelluar carcinoma and cholangiocarcinoma. Tumori 98:478–484
Lódi C, Szabó E, Holczbauer A, Batmunkh E, Szíjártó A, Kupcsulik P, Kovalszky I, Paku S, Illyés G, Kiss A, Schaff Z (2006) Claudin-4 differentiates biliary tract cancers from hepatocellular carcinomas. Mod Pathol 19:460–469
Holczbauer Á, Gyöngyösi B, Lotz G, Törzsök P, Kaposi-Novák P, Szijártó A, Tátrai P, Kupcsulik P, Schaff Z, Kiss A (2014) Increased expression of claudin-1 and claudin-7 in liver cirrhosis and hepatocellular carcinoma. Pathol Oncol Res 20:493–502
Brokalaki EI, Weber F, Sotiropoulos GC, Daoudaki M, Cicinnati VR, Beckebaum S (2012) Claudin-7 expression in hepatocellular carcinoma. Transplant Proc 44:2737–2740
Raleigh DR, Marchiando AM, Zhang Y, Shen L, Sasaki H, Wang Y, Long M, Turner JR (2010) Tight junction-associated MARVEL proteins marveld3, tricellulin, and occludin have distinct but overlapping functions. Mol Biol Cell 21:1200–1213
Murata M, Kojima T, Yamamoto T, Go M, Takano K, Osanai M, Chiba H, Sawada N (2005) Down-regulation of survival signaling through MAPK and Akt in occludin-deficient mouse hepatocytes in vitro. Exp Cell Res 310:140–151
Osanai M, Murata M, Nishikiori N, Chiba H, Kojima T, Sawada N (2006) Epigenetic silencing of occludin promotes tumorigenic and metastatic properties of cancer cells via modulations of unique sets of apoptosis-associated genes. Cancer Res 66:9125–9133
Kitajiri S, Katsuno T, Sasaki H, Ito J, Furuse M, Tsukita S (2014) Deafness in occludin-deficient mice with dislocation of tricellulin and progressive apoptosis of the hair cells. Biol Open 3:759–766
Kojima T, Sawada N (2012) Regulation of tight junctions in human normal pancreatic duct epithelial cells and cancer cells. Ann N Y Acad Sci 1257:85–92
Masuda R, Semba S, Mizuuchi E, Yanagihara K, Yokozaki H (2010) Negative regulation of the tight junction protein tricellulin by snail-induced epithelial-mesenchymal transition in gastric carcinoma cells. Pathobiology 77:106–113
Mariano C, Palmela I, Pereira P, Fernandes A, Falcão AS, Cardoso FL, Vaz AR, Campos AR, Gonçalves-Ferreira A, Kim KS, Brites D, Brito MA (2013) Tricellulin expression in brain endothelial and neural cells. Cell Tissue Res 351:397–407
Acknowledgments
This work was supported in by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labour and Welfare of Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Informed consent
Informed consent was obtained from all patients, in this study.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Additional information
Yusuke Ono and Yutaro Hiratsuka contributed equally to this work.
Rights and permissions
About this article
Cite this article
Ono, Y., Hiratsuka, Y., Murata, M. et al. Claudins-4 and -7 might be valuable markers to distinguish hepatocellular carcinoma from cholangiocarcinoma. Virchows Arch 469, 417–426 (2016). https://doi.org/10.1007/s00428-016-1984-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-016-1984-z