Abstract
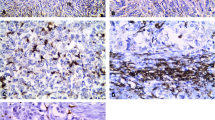
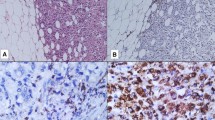
Tumor-associated macrophages play a crucial role in breast cancer progression and tumor angiogenesis. However, evaluation of tumor-associated macrophages incorporating their histological locations is lacking. The aim of this study was to clarify whether macrophages in tumor stroma and macrophages in tumor cell nests have distinctive properties in relation to pertinent breast cancer clinicopathological parameters and tumor angiogenesis. In 94 human invasive breast ductal carcinomas, tumor-associated macrophages were immunostained with anti-CD68 antibody and counted or graded according to these histological locations. Microvessels were immunostained with anti-CD34 antibody and counted for microvessel density. We found that the presence of tumor stromal and tumor nest macrophages was closely correlated (p = 0.001). Both tumor stromal and tumor nest macrophages were associated with mitotic count (p = 0.001 and p = 0.037, respectively). However, only higher tumor stromal macrophage grades were associated with higher tumor grades (p = 0.004) and negative estrogen receptor status (p = 0.007). Multivariate analysis showed that tumors with a high mitotic count score (score 3 vs. scores 1 and 2) had a higher tumor stromal macrophage density (Grades III and IV) when adjusted for tumor size, tubule formation, and estrogen receptor status (odds ratio 3.41, p = 0.010). The tumor nest macrophage count significantly correlated with the microvessel density (p < 0.001). These results imply that tumor stromal macrophages and tumor nest macrophages residing in different tumor microenvironments have distinctive roles.




Similar content being viewed by others
Abbreviations
- CI:
-
Confidence interval
- Her-2:
-
Human epidermal growth factor receptor 2
- MVD:
-
Microvessel density
- NOS:
-
Not otherwise specified
- SD:
-
Standard deviation
- TAMs:
-
Tumor-associated macrophages
References
Balkwill F, Mantovani A (2001) Inflammation and cancer: back to Virchow? Lancet 357(9255):539–545
Murri AMA, Hilmy M, Bell J, Wilson C, McNicol AM, Lannigan A, Doughty JC, McMillan DC (2008) The relationship between the systemic inflammatory response, tumour proliferative activity, T-lymphocytic and macrophage infiltration, microvessel density and survival in patients with primary operable breast cancer. Br J Cancer 99(7):1013–1019
Pupa SM, Bufalino R, Invernizzi AM, Andreola S, Rilke F, Lombardi L, Colnaghi MI, Menard S (1996) Macrophage infiltrate and prognosis in c-erbB-2-overexpressing breast carcinomas. J Clin Oncol 14(1):85–94
Lewis CE, Leek R, Harris A, McGee JO (1995) Cytokine regulation of angiogenesis in breast cancer: the role of tumor-associated macrophages. J Leukoc Biol 57(5):747–751
Lin EY, Nguyen AV, Russell RG, Pollard JW (2001) Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med 193(6):727–740
Goswami S, Sahai E, Wyckoff JB, Cammer M, Cox D, Pixley FJ, Stanley ER, Segall JE, Condeelis JS (2005) Macrophages promote the invasion of breast carcinoma cells via a colony-stimulating factor-1/epidermal growth factor paracrine loop. Cancer Res 65(12):5278–5283
Hagemann T, Robinson SC, Schulz M, Trumper L, Balkwill FR, Binder C (2004) Enhanced invasiveness of breast cancer cell lines upon co-cultivation with macrophages is due to TNF-alpha dependent up-regulation of matrix metalloproteases. Carcinogenesis 25(8):1543–1549
Volodko N, Reiner A, Rudas M, Jakesz R (1998) Tumour-associated macrophages in breast cancer and their prognostic correlations. Breast 7(2):99–105
Valkovic T, Dobrila F, Melato M, Sasso F, Rizzardi C, Jonjic N (2002) Correlation between vascular endothelial growth factor, angiogenesis, and tumor-associated macrophages in invasive ductal breast carcinoma. Virchows Arch 440(6):583–588
Jonjic N, Valkovic T, Lucin K, Iternicka Z, Krstulja M, Mustac E, Dobi-Babic R, Sasso F, Melato M (1998) Comparison of microvessel density with tumor associated macrophages in invasive breast carcinoma. Anticancer Res 18(5B):3767–3770
Tsutsui S, Yasuda K, Suzuki K, Tahara K, Higashi H, Era S (2005) Macrophage infiltration and its prognostic implications in breast cancer: the relationship with VEGF expression and microvessel density. Oncol Rep 14(2):425–431
Leek RD, Lewis CE, Whitehouse R, Greenall M, Clarke J, Harris AL (1996) Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res 56(20):4625–4629
Valentin G, Laura B, Marina Z, Hellmut GA (1999) Induction of inflammatory angiogenesis by monocyte chemoattractant protein-1. Int J Cancer 82(5):765–770
Bolat F, Kayaselcuk F, Nursal TZ, Yagmurdur MC, Bal N, Demirhan B (2006) Microvessel density, VEGF expression, and tumor-associated macrophages in breast tumors: correlations with prognostic parameters. J Exp Clin Cancer Res 25(3):365–372
Sunderkotter C, Steinbrink K, Goebeler M, Bhardwaj R, Sorg C (1994) Macrophages and angiogenesis. J Leukoc Biol 55(3):410–422
Crowther M, Brown NJ, Bishop ET, Lewis CE (2001) Microenvironmental influence on macrophage regulation of angiogenesis in wounds and malignant tumors. J Leukoc Biol 70(4):478–490
Craig M, Claire EL (2005) Macrophage migration and gene expression in response to tumor hypoxia. Int J Cancer 117(5):701–708
Lin EY, Li J-F, Gnatovskiy L, Deng Y, Zhu L, Grzesik DA, Qian H, X-n X, Pollard JW (2006) Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res 66(23):11238–11246
Ch’ng ES, Jaafar H, Tuan Sharif SE (2011) Breast tumor angiogenesis and tumor-associated macrophages: histopathologist’s perspective. Patholog Res Int 2011(2011):572706
Valkovic T, Fuckar D, Stifter S, Matusan K, Hasan M, Dobrila F, Jonjic N (2005) Macrophage level is not affected by monocyte chemotactic protein-1 in invasive ductal breast carcinoma. J Cancer Res Clin Oncol 131(7):453–458
Lee AH, Happerfield LC, Bobrow LG, Millis RR (1997) Angiogenesis and inflammation in invasive carcinoma of the breast. J Clin Pathol 50(8):669–673
Leek RD, Landers RJ, Harris AL, Lewis CE (1999) Necrosis correlates with high vascular density and focal macrophage infiltration in invasive carcinoma of the breast. Br J Cancer 79(5–6):991–995
Ueno T, Toi M, Saji H, Muta M, Bando H, Kuroi K, Koike M, Inadera H, Matsushima K (2000) Significance of macrophage chemoattractant protein-1 in macrophage recruitment, angiogenesis, and survival in human breast cancer. Clin Cancer Res 6(8):3282–3289
Leek RD, Hunt NC, Landers RJ, Lewis CE, Royds JA, Harris AL (2000) Macrophage infiltration is associated with VEGF and EGFR expression in breast cancer. J Pathol 190(4):430–436
Hisashi S, Morio K, Takao Y, Shigehira S, Motoharu S, Kouji M, Masakazu T (2001) Significant correlation of monocyte chemoattractant protein-1 expression with neovascularization and progression of breast carcinoma. Cancer 92(5):1085–1091
Lewis JS, Landers RJ, Underwood JCE, Harris AL, Lewis CE (2000) Expression of vascular endothelial growth factor by macrophages is up-regulated in poorly vascularized areas of breast carcinomas. J Pathol 192(2):150–158
Hiroshi F, Takafumi S, Genichiro I, Akashi I, Takeshi N, Masaru M, Atsushi O (2009) Stromal MCP-1 in mammary tumors induces tumor-associated macrophage infiltration and contributes to tumor progression. Int J Cancer 125(6):1276–1284
Vicioso L, Gonzalez FJ, Alvarez M, Ribelles N, Molina M, Marquez A, Perez L, Matilla A, Alba E (2006) Elevated serum levels of vascular endothelial growth factor are associated with tumor-associated macrophages in primary breast cancer. Am J Clin Pathol 125(1):111–118
Ohno S, Inagawa H, Soma G, Nagasue N (2002) Role of tumor-associated macrophage in malignant tumors: should the location of the infiltrated macrophages be taken into account during evaluation? Anticancer Res 22(6C):4269–4275
Elston CW, Ellis IO (1991) pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 19(5):403–410
Dako (2010) HercepTest™ interpretation manual — breast.
Weidner N, Semple JP, Welch WR, Folkman J (1991) Tumor angiogenesis and metastasis—correlation in invasive breast carcinoma. N Engl J Med 324(1):1–8
Fox SB (2006) Quantitative angiogenesis in breast cancer. In: Brooks SA, Harris A (eds) Breast cancer research protocols. Methods in molecular medicine. Humana Press, Totowa, NJ, pp 161–187
Ch’ng ES, Tuan Sharif SE, Jaafar H (2012) Characteristics of invasive breast ductal carcinoma, NOS, diagnosed in a tertiary institution in the East Coast of Malaysia with a focus on tumor angiogenesis. Asian Pac J Cancer Prev 13(9):4445–4452
Ribatti D, Nico B, Crivellato E, Vacca A (2007) Macrophages and tumor angiogenesis. Leukemia 21(10):2085–2089
Stossi F, Madak-Erdogan Z, Katzenellenbogen BS (2012) Macrophage-elicited loss of estrogen receptor-[alpha] in breast cancer cells via involvement of MAPK and c-Jun at the ESR1 genomic locus. Oncogene 31(14):1825–1834
Harkonen PL, Vaananen HK (2006) Monocyte–macrophage system as a target for estrogen and selective estrogen receptor modulators. Ann N Y Acad Sci 1089(1):218–227
Hammond MEH, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M (2010) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version). Arch Pathol Lab Med 134(7):48–72
Iwamoto T, Booser D, Valero V, Murray JL, Koenig K, Esteva FJ, Ueno NT, Zhang J, Shi W, Qi Y, Matsuoka J, Yang EJ, Hortobagyi GN, Hatzis C, Symmans WF, Pusztai L (2012) Estrogen Receptor (ER) mRNA and ER-related gene expression in breast cancers that are 1 % to 10 % ER-positive by immunohistochemistry. J Clin Oncol 30(7):729–734
Deyarmin B, Kane J, Valente A, Laar R, Gallagher C, Shriver C, Ellsworth R (2013) Effect of ASCO/CAP guidelines for determining ER status on molecular subtype. Ann Surg Oncol 20(1):87–93
Lewis CE, Pollard JW (2006) Distinct role of macrophages in different tumor microenvironments. Cancer Res 66(2):605–612
Acknowledgements
This work was supported by Universiti Sains Malaysia Short Term Grant (304/PPSP/61310048).
Conflict of interests
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ch’ng, E.S., Tuan Sharif, S.E. & Jaafar, H. In human invasive breast ductal carcinoma, tumor stromal macrophages and tumor nest macrophages have distinct relationships with clinicopathological parameters and tumor angiogenesis. Virchows Arch 462, 257–267 (2013). https://doi.org/10.1007/s00428-012-1362-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-012-1362-4