Abstract
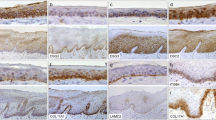
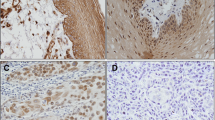
Desmocollin 3 (Dsc3) and desmoglein 3 (Dsg3) are both transmembrane glycoproteins that belong to the cadherin family of calcium-dependent cell adhesion molecules. β-Catenin is a member of the cadherin–catenin complex that mediates homotypic cell–cell adhesion and is also an important molecule in the wnt signaling pathway. In this study, we examined the simultaneous expression level of Dsc3, Dsg3, and β-catenin in oral squamous cell carcinomas (OSCCs) and normal oral epithelia using immunohistochemistry. There was a significant correlation (p < 0.05) among the following variables in OSCCs: reduced or loss of expression of Dsc3, Dsg3, and β-catenin compared to normal oral epithelium, reduced or loss of expression of Dsc3 and histological grade (moderately or poorly differentiated), and reduced or loss of expression of β-catenin and lymph node metastasis. Furthermore, a positive correlation was found between reduced or loss of β-catenin staining and reduced or loss of Dsc3 staining in lymph node metastatic cancer tissue (r = 0.734, p < 0.05). These results suggest an abnormal expression of Dsc3, Dsg3, and β-catenin induced in the progression of oral carcinomas and that the Dsc3 expression level might be related to the regulation of β-catenin in lymph node metastasis and cell proliferation in OSCCs.



Similar content being viewed by others
References
Alazawi WO, Morris LS, Stanley MA, Garrod DR, Coleman DR (2003) Altered expression of desmosomal components in high-grade squamous intraepithelial lesions of the cervix. Virchows Arch 443:51–56
Barth AI, Nathke IS, Nelson WJ (1997) Cadherins, catenins and APC protein: interplay between cytoskeletal complexes and signaling pathways. Curr Opin Cell Biol 9:683–690
Brasanac D, Boricic I, Todorovic V, Tomanovic N, Radojevic S (2005) Cyclin A and beta-catenin expression in actinic keratosis, Bowen’s disease and invasive squamous cell carcinoma of the skin. Br J Dermatol 153:1166–1175
Chen X, Bonne S, Hatzfeld M, van Roy F, Green KJ (2002) Protein binding and functional characterization of plakophilin 2. Evidence for its diverse roles in desmosomes and beta-catenin signaling. J Biol Chem 277:10512–10522
Chidgey M (2002) Desmosomes and disease: an update. Histol Histopathol 17:1179–1192
Conacci-Sorrell M, Zhurinsky J, Ze’ev B (2002) The cadherin–catenin adhesion system in signalling and cancer. J Clin Invest 109:987–991
Cui J, Zhou XD, Liu YK, Tang ZY, Zile MH (2001) Abnormal beta-catenin gene expression with invasiveness of primary hepatocellular carcinoma in China. World J Gastroenterol 7:542–546
Damjanov I, Fan F (2007) Tumors of the mouth, pharynx, nose, and paranasal sinuses. In: Damjanov I, Fan F (eds) Cancer grading manual. Springer, New York, pp 6–12
Depondt J, Shabana AH, Florescu-Zorila S, Gehanno P, Forest N (1999) Down-regulation of desmosomal molecules in oral and pharyngeal squamous cell carcinomas as a marker for tumour growth and distant metastasis. Eur J Oral Sci 107:183–193
Garrod DR (1995) Desmosomes and cancer. Cancer Surv 24:97–111
Garrod DR, Merritt AJ, Nie Z (2002) Desmosomal cadherins. Curr Opin Cell Biol 14:537–545
Getsios S, Huen AC, Green KJ (2004) Working out the strength and flexigility of desmosomes. Nat Rev Mol Cell Biol 5:271–281
Hanakawa Y, Amagai M, Shirakata Y, Sayama K, Hashimoto K (2000) Different effects of dominant negative mutants of desmocollin and desmoglein on the cell–cell adhesion of keratinocytes. J Cell Sci 113:1803–1811
Hardman MJ, Liu K, Avilion AA, Merritt A, Brennan K, Garrod DR, Byrne C (2005) Desmosomal cadherin misexpression alters beta-catenin stability and epidermal differentiation. Mol Cell Biol 25:969–978
Hiraki A, Shinohara M, Ikebe T, Nakamura S, Kurahara S, Garrod DR (1996) Immunohistochemical staining of desmosomal components in oral squamous cell carcinomas and its association with tumour behaviour. Br J Cancer 73:1491–1497
Hoschuetzky H, Aberle H, Kemler R (1994) Beta-catenin mediates the interaction of the cadherin–catenin complex with epidermal growth factor receptor. J Cell Biol 127:1375–1380
Hynes RO (1992) Integrins: versatility, modulation, and signaling in cell adhesion. Cell 69:11–25
Iwai S, Katagiri W, Kong C, Amekawa S, Nakazawa M, Yura Y (2005) Mutations of the APC, beta-catenin, and axin 1 genes and cytoplasmic accumulation of beta-catenin in oral squamous cell carcinoma. J Cancer Res Clin Oncol 131:773–782
Karayiannakis AJ, Nakopoulou L, Gakiopoulou H, Keramopoulos A, Davaris PS, Pignatelli M (2001) Expression patterns of beta-catenin in in situ and invasive breast cancer. Eur J Surg Oncol 27:31–36
Krunic AL, Garrod DR, Madani S, Buchanan MD, Clark RE (1998) Immunohistochemical staining for desmogleins 1 and 2 in keratinocytic neoplasms with squamous phenotype: actinic keratosis, keratoacanthoma and squamous cell carcinoma of the skin. Br J Cancer 77:1275–1279
Krunic AL, Garrod DR, Smith NP, Orchard GS, Cvijetic OB (1996) Differential expression of desmosomal glycoproteins in keratoacanthoma and squamous cell carcinoma of the skin: an immunohistochemical aid to diagnosis. Acta Derm-Venereol 76:394–398
Kurzen H, Munzing I, Hartschuh W (2003) Expression of desmosomal proteins in squamous cell carcinomas of the skin. J Cutan Pathol 30:621–630
Natsugoe S, Nakashima S, Matsumoto M, Sakita H, Sakamoto F, Okumura H, Baba M, Yoshinaka H, Takao S, Aikou T (2002) Biologic and imaging diagnosis of lymph node metastasis in esophageal carcinoma. J Surg Oncol 81:25–32
Nelson WJ, Nusse R (2004) Convergence of Wnt, beta-catenin, and cadherin pathways. Science 303:1483–1487
Ochiai A, Akimoto S, Shibata T, Kanai Y, Shibata T, Oyama T, Hirohashi S (1994) c-erbB2 gene product associates with catenins in human cancer cells. Biochem Biophys Res Commun 205:73–78
Peifer M, Berg S, Reynolds AB (1994) A repeating amino acid motif shared by proteins with diverse cellular roles. Cell 76:789–791
Peifer M, McCrea PD, Green KJ, Wieschaus E, Gumbiner BM (1992) The vertebrate adhesive junction proteins beta-catenin and plakoglobin and the Drosophila segment polarity gene armadillo form a multigene family with similar properties. J Cell Biol 118:681–691
Pindborg JJ, Reichart PA, Smith CJ, van der Waal I (1997) Histological typing of cancer and precancer of the oral mucosa. Springer, Berlin
Pirinen RT, Hirvikoski P, Johansson RT, Hollmen S, Kosma VM (2001) Reduced expression of alpha-catenin, beta-catenin, and gamma-catenin is associated with high cell proliferative activity and poor differentiation in non-small cell lung cancer. J Clin Pathol 54:391–395
Pittella F, Katsube K, Takemura T, Hashimoto T, Kawano T, Garrod D, Takagi M (2001) Perinuclear and cytoplasmic distribution of desmoglein in esophageal squamous cell carcinomas. Pathol Res Pract 197:85–91
Qiao Q, Ramadani M, Gansauge S, Gansauge F, Leder G, Beger HG (2001) Reduced membranous and ectopic cytoplasmic expression of beta-catenin correlate with cyclin D1 overexpression and poor prognosis in pancreatic cancer. Int J Cancer 95:194–197
Saldanha G, Ghura V, Potter L, Fletcher A (2004) Nuclear beta-catenin in basal cell carcinoma correlates with increased proliferation. Br J Dermatol 151:157–164
Sawa Y, Kuroshima S, Yamaoka Y, Yoshida S (2005) Intracellular distribution of desmoplakin in human odontoblasts. J Histochem Cytochem 53:1099–1108
Shinohara M, Hiraki A, Ikebe T, Nakamura S, Kurahara S, Shirasuna K, Garrod DR (1998) Immunohistochemical study of desmosomes in oral squamous cell carcinoma: correlation with cytokeratin and E-cadherin staining, and with tumour behaviour. J Pathol 184:369–381
Su LK, Vogelstein B, Kinzler KW (1993) Association of the APC tumour suppressor protein with catenins. Science 262:1734–1737
Williams HK, Sanders DS, Jankowski JA, Landini G, Brown AM (1998) Expression of cadherins and catenins in oral epithelial dysplasia and squamous cell carcinoma. J Oral Pathol Med 27:308–317
Yeh KT, Chang JG, Lin TH, Wang YF, Chang JY, Shih MC, Lin CC (2003) Correlation between protein expression and epigenetic mutation changes of Wnt pathway-related genes in oral cancer. Int J Oncol 23:1001–1007
Yin T, Green KJ (2004) Regulation of desmosome assembly and adhesion. Semin Cell Dev Biol 15:665–677
Zhurinsky J, Shtutman M, Ben-Ze'ev A (2000) Plakoglobin and beta-catenin: protein interactions, regulation and biological roles. J Cell Sci 113:3127–3131
Acknowledgments
The authors thank members of Oral Pathology, Graduate School of Tokyo Medical and Dental University for their excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, L., Liu, T., Wang, Y. et al. Altered expression of desmocollin 3, desmoglein 3, and β-catenin in oral squamous cell carcinoma: correlation with lymph node metastasis and cell proliferation. Virchows Arch 451, 959–966 (2007). https://doi.org/10.1007/s00428-007-0485-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-007-0485-5