Abstract
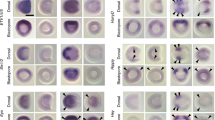
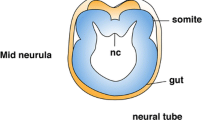
In the basal chordate amphioxus (Branchiostoma), somites extend the full length of the body. The anteriormost somites segment during the gastrula and neurula stages from dorsolateral grooves of the archenteron. The remaining ones pinch off, one at a time, from the tail bud. These posterior somites appear to be homologous to those of vertebrates, even though the latter pinch off from the anterior end of bands of presomitic mesoderm rather than directly from the tail bud. To gain insights into the evolution of mesodermal segmentation in chordates, we determined the expression of ten genes in nascent amphioxus somites. Five (Uncx4.1, NeuroD/atonal-related, IrxA, Pcdhδ2-17/18, and Hey1) are expressed in stripes in the dorsolateral mesoderm at the gastrula stage and in the tail bud while three (Paraxis, Lcx, and Axin) are expressed in the posterior mesendoderm at the gastrula and neurula stages and in the tail bud at later stages. Expression of two genes (Pbx and OligA) suggests roles in the anterior somites that may be unrelated to initial segmentation. Together with previous data, our results indicate that, with the exception that Engrailed is only segmentally expressed in the anterior somites, the genetic mechanisms controlling formation of both the anterior and posterior somites are probably largely identical. Thus, the fundamental pathways for mesodermal segmentation involving Notch–Delta, Wnt/β-catenin, and Fgf signaling were already in place in the common ancestor of amphioxus and vertebrates although budding of somites from bands of presomitic mesoderm exhibiting waves of expression of Notch, Wnt, and Fgf target genes was likely a vertebrate novelty. Given the conservation of segmentation gene expression between amphioxus and vertebrate somites, we propose that the clock mechanism may have been established in the basal chordate, while the wavefront evolved later in the vertebrate lineage.




Similar content being viewed by others
References
Aulehla A, Wiegraebe W, Baubet V, Wahl MB, Deng C, Taketo M, Lewandoski M, Pourquié (2008) A β-catenin gradient links the clock and wavefront systems in mouse embryo segmentation. Nat Cell Biol 10:186–193
Beaster-Jones L, Horton AC, Gibson-Brown JJ, Holland ND, Holland LZ (2006) The amphioxus T-box gene, AmphiTbx15/18/22, illuminates the origins of chordate segmentation. Evol Dev 8:119–129
Boronchain OJ, Pollet N, Ymlahi-Ouazzani Q, Dhorne-Pollet S, Heibling JC, Lecarpentier JE, Percheron K, Wegnez M (2007) The olig family: phylogenetic analysis and early gene expression in Xenopus tropicalis. Dev Genes Evol 217:485–497
Burgess R, Rawls A, Brown D, Bradley A, Olson EN (1996) Requirement of the paraxis gene for somite formation and musculoskeletal patterning. Nature 384:570–573
Bussen M, Petry M, Schuster-Gossler K, Leitges M, Gossler A, Kispert A (2004) The T-box transcription factor Tbx18 maintains the separation of anterior and posterior somite compartments. Genes Dev 18:1209–1221
Cinquin O (2007) Understanding the somitogenesis clock: what’s missing? Mech Dev 124:501–517
Dequeant ML, Glynn E, Gaudenz K, Wahl M, Chen J, Mushegian A, Pourquié O (2006) A complex oscillating network of signaling genes underlies the mouse segmentation clock. Science 314:1595–1598
Dunty WC Jr, Biris KK, Chalamalasetty RB, Taketo MM, Lewandoski M, Yamaguchi TP (2008) Wnt3a/-catenin signaling controls posterior body development by coordinating mesoderm formation and segmentation. Development 135:85–94
Elmasri H, Liedtke D, Lucking G, Volff J-N, Gessler M, Winkler C (2004) her7 and hey1, but not lunatic fringe show dynamic expression during somitogenesis in medaka (Oryzias latipes). Gene Exp Patterns 4:553–559
Farin HF, Bussen M, Schmidt MK, Singh MK, Schuster-Gossler K, Kispert A (2007) Transcriptional repression by the T-box proteins Tbx18 and Tbx15 depends on groucho corepressors. J Biol Chem 282:25748–25759
Feller J, Schneider A, Schuster-Gossler K, Gossler A (2008) Noncyclic Notch activity in the presomitic mesoderm demonstrates uncoupling of somite compartmentalization and boundary formation. Genes Dev 22:2166–2171
Flood PR (1975) Fine structure of the notochord of amphioxus. Symp Zool Soc London 36:81–104
Goldbeter A, Pourquie O (2008) Modeling the segmentation clock as a network of coupled oscillations in the Notch, Wnt and FGF signaling pathways. J Theor Biol 252:574–585
Holland LZ, Holland ND (2000) Developmental expression of AmphiWnt1, an amphioxus gene in the Wnt1/wingless subfamily. Dev Genes Evol 210:522–524
Holland LZ, Yu JK (2004) Cephalochordate (Amphioxus) embryos: procurement, culture, basic methods. Methods Cell Biol 74:195–215
Holland LZ, Holland PWH, Holland ND (1996) Revealing homologies between body parts of distantly related animals by in situ hybridization to developmental genes: amphioxus versus vertebrates. In: Palumbi S, Ferraris JD (eds) Molecular approaches to zoology and evolution. Wiley, New York, pp 267–282, 473–483
Holland LZ, Kene M, Williams N, Holland ND (1997) Sequence and embryonic expression of the amphioxus engrailed gene (AmphiEn): the metameric pattern of transcription resembles that of its segment-polarity homolog in Drosophila. Development 124:1723–1732
Holland LZ, Rached LA, Tamme R, Holland ND, Inoko H, Shiina T, Burgtorf C, Lardelli M (2001) Characterization and developmental expression of the amphioxus homolog of Notch (AmphiNotch): evolutionary conservation of multiple expression domains in amphioxus and vertebrates. Dev Biol 232:493–507
Holland LZ, Panfilio KA, Chastain R, Schubert M, Holland ND (2005) Nuclear beta-catenin promotes non-neural ectoderm and posterior cell fates in amphioxus embryos. Dev Dyn 233:1430–1443
Holland LZ, Holland ND, Gilland E (2008) Amphioxus and the evolution of head segmentation. Integrative Comp Biol. doi:10.1093/icb/icn060
Homayouni R, Rice DS, Curran T (2001) Disabled-1 interacts with a novel developmentally regulated protocadherin. Biochem Biophys Res Comm 289:539–547
Hoppler S, Brown JD, Moon RT (1996) Expression of a dominant-negative Wnt blocks induction of MyoD in Xenopus embryos. Genes Dev 10:2805–2817
Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F (2002) Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol 22:1172–1183
Kawamura A, Koshida S, Takada S (2008) Activator-to-repressor conversion of T-box transcription factors by the Ripply family of Groucho/TLE-associated mediators. Mol Cell Biol 28:3236–3244. doi:10.1128/MCB.01754-07
Kim S, Yamamoto A, Bouwmeester T, Agius E, Robertis E (1998) The role of paraxial protocadherin in selective adhesion and cell movements of the mesoderm during Xenopus gastrulation. Development 125:4681–4690
Kim S-H, Jen W-C, De Robertis EM, Kintner C (2000) The protocadherin PAPC establishes segmental boundaries during somitogenesis in Xenopus embryos. Current Biol 10:821–830
Kim S-Y, Chung HS, Sun W, Kim H (2007) Spatiotemporal expression pattern of non-clustered protocadherin family members in the developing rat brain. Neuroscience 147:996–1021
Kozmik Z, Holland ND, Kreslova J, Oliveri D, Schubert M, Jonasova K, Holland LZ, Pestarino M, Benes V, Candiani S (2007) Pax-Six-Eya-Dach network during amphioxus development: conservation in vitro but context specificity in vivo. Dev Biol 306:143–159
Kusakabe R, Kuratani S (2007) Evolutionary perspectives from development of mesodermal components in the lamprey. Dev Dyn 236:2410–2420
Kusakabe R, Kusakabe T, Satoh N, Holland ND, Holland LZ (1997) Differential gene expression and intracellular mRNA localization of amphioxus actin isoforms throughout development: implications for conserved mechanisms of chordate development. Dev Genes Evol 207:203–215
Laurent A, Bihan R, Omilli F, Deschamps S, Pellerin I (2008) PBX proteins: much more than Hox cofactors. Dev Biol 52:9–20
Lebel M, Agarwal P, Cheng CW, Kabir MG, Chan TY, Thanabalasingham V, Zhang X, Cohen DR, Husain M, Cheng SH, Bruneau BG, Hui C-C (2003) The Iroquois homeobox gene Irx2 is not essential for normal development of the heart and midbrain-hindbrain boundary in mice. Mol Cell Biol 23:8216–8225
Leimeister C, Dale K, Fischer A, Klamt B, Hrabe de Angelis M, Radtke F, McGrew MJ, Pourquie O, Gessler M (2000) Oscillating expression of c-Hey2 in the presomitic mesoderm suggests that the segmentation clock may use combinatorial signaling through multiple interacting bHLH factors. Dev Biol 227:91–103
Li X, Zhang W, Chen D, Lin Y, Huang X, Shi D, Zhang H (2006) Expression of a novel somite-formation-related gene, AmphiSom, during amphioxus development. Dev Genes Evol 216:52–55
Linker C, Lesbros C, Gros J, Burrus LW, Rawls A, Marcelle C (2005) β-catenin-dependent Wnt signalling controls the epithelial organisation of somites through the activation of paraxis. Development 132:3895–3905
Mara A, Holley SA (2007) Oscillators and the emergence of tissue organization during zebra fish somitogenesis. Trends Cell Biol 17:593–599
Matsumoto K, Nishihara S, Kamimura M, Shiraishi T, Otoguro T, Uehara M, Maeda Y, Ogura K, Lumsden A, Ogura T (2004) The prepattern transcription factor Irx2, a target of the FGF8/MAP kinase cascade, is involved in cerebellum formation. Nature Neurosci 7:605–612
Meulemans D, Bronner-Fraser M (2007) Insights from amphioxus into the evolution of vertebrate cartilage. PLoS ONE 2:e787
Minguillón C, Jimenez-Delgado S, Panopoulou G, Garcia-Fernandez J (2003) The amphioxus Hairy family: differential fate after duplication. Development 130:5903–5914
Morimoto M, Sasaki N, Oginuma M, Kiso M, Igarashi K, Aizaki K, Kanno J, Saga Y (2007) The negative regulation of Mesp2 by mouse Ripply2 is required to establish the rostro-caudal patterning within a somite. Development 134:1561–1569
Neal HV (1918) The history of the eye muscles. J Morphol 30:433–453
Noden DM, Francis-West P (2006) The differentiation and morphogenesis of craniofacial muscles. Dev Dynam 235:1194–1218
Noden DM, Trainor PA (2005) Relations and interactions between cranial mesoderm and neural crest populations. J Anat 207:575–601
Offner N, Dubal N, Jamrich M, Durand B (2005) The pro-apoptotic activity of a vertebrate Bar-like homeobox gene plays a key role in patterning the Xenopus neural plate by limiting the number of chordin-and shh-expressing cells. Development 132:1807–1818
Őzbudak E, Pourquié O (2008) The vertebrate segmentation clock: the tip of the iceberg. Curr Opin Genet Dev. doi:10.1016/j.gde.2008.06.007
Rasmussen SLK, Holland LZ, Schubert M, Beaster-Jones L, Holland ND (2007) Amphioxus AmphiDelta: evolution of delta protein structure, segmentation, and neurogenesis. Genesis 45:113–122
Redies C, Vanhalst K, van Roy F (2005) δ-Protocadherins: unique structures and functions. Cell Mol Life Sci 62:2840–2852
Reig G, Cabrejos ME, Concha ML (2007) Functions of BarH transcription factors during embryonic development. Dev Biol 302:367–375
Schragle J, Huang R, Christ B, Prols F (2004) Control of the temporal and spatial Uncx4.1 expression in the paraxial mesoderm of avian embryos. Anat Embryol. 208:323–332
Schubert M, Holland LZ, Panopoulou GD, Lehrach H, Holland ND (2000) Characterization of amphioxus AmphiWnt8: insights into the evolution of patterning of the embryonic dorsoventral axis. Evol Dev 2:85–92
Schubert M, Holland LZ, Stokes MD, Holland ND (2001) Three amphioxus Wnt genes (AmphiWnt3, AmphiWnt5, and AmphiWnt6) associated with the tail bud: the evolution of somitogenesis in chordates. Dev Biol 240:262–273
Schubert M, Yu JK, Holland ND, Escriva H, Laudet V, Holland LZ (2005) Retinoic acid signaling acts via Hox1 to establish the posterior limit of the pharynx in the chordate amphioxus. Development 132:61–73
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Wang Y, Janicki P, Koster I, Berger CD, Wenzl C, Grosshans J, Steinbeisser H (2008) Xenopus paraxial protocadherin regulates morphogenesis by antagonizing sprouty. Genes Dev 22:878–883
Yu J-K, Satou Y, Holland ND, Shin-i T, Kohara Y, Satoh N, Bronner-Fraser M, Holland LZ (2007) Axial patterning in cephalochordates and the evolution of the organizer. Nature 445:613–617
Yu J-K, Satou Y, Wang M-C, Shin I-T, Kohara Y, Holland LZ, Satoh N (2008) A cDNA resource for the cephalochordate amphioxus Branchiostoma floridae. Dev Genes Evol. doi:10.1007/s00427-008-0228-x
Acknowledgements
We thank John Lawrence, Susan Bell, and the staff of the University of South Florida for providing laboratory space during the summer breeding season of amphioxus. This work was supported by an National Science Fund (NSF) predoctoral fellowship to S. L. Kaltenbach and by NSF grants Grant IBN 02-3617 to L. Z. H. and IBN 04-16292 to L. Z. H. and N. D. Holland.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Gibson-Brown
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 278 kb)
ESM Table 1
EST clones used to synthesize probes for in situ hybridization (DOC 29.0 kb)
ESM Table 2
Protein sequences (DOC 26.5 kb)
Rights and permissions
About this article
Cite this article
Beaster-Jones, L., Kaltenbach, S.L., Koop, D. et al. Expression of somite segmentation genes in amphioxus: a clock without a wavefront?. Dev Genes Evol 218, 599–611 (2008). https://doi.org/10.1007/s00427-008-0257-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00427-008-0257-5