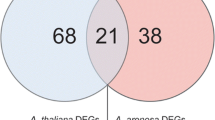
Abstract
Main conclusion
Altered expression of the TaRSL2 gene was positively correlated with variation in root hair length during allopolyploid wheat evolution, and overexpression of TaRSL2 in Arabidopsis increases root hair length.
Root hairs aid nutrient and water uptake and anchor the plant in the soil. Allopolyploid wheats display significant growth vigor in terms of root hair length compared to their diploid progenitors, but little is known about the molecular basis of variation in root hair length during wheat allopolyploidization. Here, we isolated three orthologs of the Arabidopsis root hair gene ROOT HAIR DEFECTIVE SIX-LIKE 2 (AtRSL2) in allohexaploid wheat, designated TaRSL2-4A, TaRSL2-4B and TaRSL2-4D. The deduced polypeptides of these three TaRSL2 homoeologous genes shared high similarity, and a conserved basic helix-loop-helix (bHLH) domain was present in their C-terminal regions. Notably, the expression of TaRSL2 was positively correlated with root hair length of wheat accessions with different ploidy levels. Moreover, ectopic overexpression of TaRSL2-4D in Arabidopsis could increase root hair length. We found that the transcript levels of TaRSL2 homoeologous genes dynamically changed during allopolyploid wheat evolution, implicating the complexity of the underlying molecular mechanism. Collectively, we propose that altered expression of the TaRSL2 gene contributed to variation in root hair length in allopolyploid wheats.






Similar content being viewed by others
Abbreviations
- bHLH:
-
Basic helix-loop-helix
- RSL:
-
ROOT HAIR DEFECTIVE SIX-LIKE
References
Akhunova AR, Matniyazov RT, Liang HQ, Akhunov ED (2010) Homoeolog-specific transcriptional bias in allopolyploid wheat. BMC Genom 11:505. doi:10.1186/1471-2164-11-505
Balao F, Herrera J, Talavera S (2011) Phenotypic consequences of polyploidy and genome size at the microevolutionary scale: a multivariate morphological approach. N Phytol 192:256–265
Brown LK, George TS, Thompson JA, Wright G, Lyon J, Dupuy L, Hubbard SF, White PJ (2012) What are the implications of variation in root hair length on tolerance to phosphorus deficiency in combination with water stress in barley (Hordeum vulgare)? Ann Bot 110:319–328
Bruex A, Kainkaryam RM, Wieckowski Y, Kang YH, Bernhardt C, Xia Y, Zheng XH, Wang JY, Lee MM, Benfey P, Woolf PJ, Schiefelbein J (2012) A gene regulatory network for root epidermis cell differentiation in Arabidopsis. PLoS Genet 8(1):e1002446. doi:10.1371/journal.pgen.1002446
Chen ZJ (2007) Genetic and epigenetic mechanisms for gene expression and phenotypic variation in plant polyploids. Annu Rev Plant Biol 58:377–406
Clarke JD (2009) Cetyltrimethyl ammonium bromide (CTAB) DNA miniprep for plant DNA isolation. Cold Spring Harbor Protocols 2009, pdb. prot5177
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743
Datta S, Kim CM, Pernas M, Pires ND, Proust H, Tam T, Vijayakumar P, Dolan L (2011) Root hairs: development, growth and evolution at the plant-soil interface. Plant Soil 346:1–14
Dubcovsky J, Dvorak J (2007) Genome plasticity a key factor in the success of polyploid wheat under domestication. Science 316:1862–1866
Feldman M, Levy AA (2005) Allopolyploidy-a shaping force in the evolution of wheat genomes. Cytogenet Genome Res 109:250–258
Feldman M, Lupton FGH, Miller TE (1995) Wheats. In: Smartt J, Simmonds NW (eds) Evolution of crop plants, 2nd edn. Longman Scientific, London, pp 184–192
Feldman M, Levy AA, Fahima T, Korol A (2012) Genomic asymmetry in allopolyploid plants: wheat as a model. J Exp Bot 63:5045–5059
Franciosini A, Rymen B, Shibata M, Favero DS, Sugimoto K (2017) Molecular networks orchestrating plant cell growth. Curr Opin Plant Biol 35:98–104
Gilroy S, Jones DL (2000) Through form to function: root hair development and nutrient uptake. Trends Plant Sci 5:56–60
Haling RE, Brown LK, Bengough AG, Young IM, Hallett PD, White PJ, George TS (2013) Root hairs improve root penetration, root-soil contact, and phosphorus acquisition in soils of different strength. J Exp Bot 64:3711–3721
Han Y, Xin M, Huang K, Xu Y, Liu Z, Hu Z, Yao Y, Peng H, Ni Z, Sun Q (2016) Altered expression of TaRSL4 gene by genome interplay shapes root hair length in allopolyploid wheat. N Phytol 209:721–732
He X, Zeng J, Cao F, Ahmed IM, Zhang G, Vincze E, Wu F (2015) HvEXPB7, a novel beta-expansin gene revealed by the root hair transcriptome of Tibetan wild barley, improves root hair growth under drought stress. J Exp Bot 66:7405–7419
Horn R, Wingen LU, Snape JW, Dolan L (2016) Mapping of quantitative trait loci for root hair length in wheat identifies loci that co-locate with loci for yield components. J Exp Bot 67:4535–4543
Huang S, Sirikhachornkit A, Su X, Faris J, Gill B, Haselkorn R, Gornicki P (2002) Genes encoding plastid acetyl-CoA carboxylase and 3-phosphoglycerate kinase of the Triticum/Aegilops complex and the evolutionary history of polyploid wheat. Proc Natl Acad Sci USA 99:8133–8138
Hwang Y, Choi HS, Cho HM, Cho HT (2017) Tracheophytes contain conserved orthologs of a basic helix-loop-helix transcription factor to modulate ROOT HAIR SPECIFIC genes. Plant Cell 29:39–53
Jackson S, Chen ZJ (2010) Genomic and expression plasticity of polyploidy. Curr Opin Plant Biol 13:153–159
Kim CM, Dolan L (2016) ROOT HAIR DEFECTIVE SIX-LIKE Class I genes promote root hair development in the grass Brachypodium distachyon. PLoS Genet 12:e1006211. doi:10.1371/journal.pgen.1006211
Kim DW, Lee SH, Choi SB, Won SK, Heo YK, Cho M, Park YI, Cho HT (2006) Functional conservation of a root hair cell-specific cis-element in angiosperms with different root hair distribution patterns. Plant Cell 18:2958–2970
Kim CM, Han CD, Dolan L (2017) RSL class I genes positively regulate root hair development in Oryza sativa. N Phytol 213:314–323
Kwasniewski M, Nowakowska U, Szumera J, Chwialkowska K, Szarejko I (2013) iRootHair: a comprehensive root hair genomics database. Plant Physiol 161:28–35
Li A, Liu D, Wu J, Zhao X, Hao M, Geng S, Yan J, Jiang X, Zhang L, Wu J, Yin L, Zhang R, Wu L, Zheng Y, Mao L (2014) mRNA and small RNA transcriptomes reveal insights into dynamic homoeolog regulation of allopolyploid heterosis in nascent hexaploid wheat. Plant Cell 26:1878–1900
Liu B, Xu CM, Zhao N, Qi B, Kimatu JN, Pang JS, Han FP (2009) Rapid genomic changes in polyploid wheat and related species: implications for genome evolution and genetic improvement. J Genet Genom 36:519–528
Ma Z, Bielenberg DG, Brown KM, Lynch JP (2001) Regulation of root hair density by phosphorus availability in Arabidopsis thaliana. Plant Cell Environ 24:459–467
Madlung A (2013) Polyploidy and its effect on evolutionary success: old questions revisited with new tools. Heredity 110:99–104
Marzec M, Melzer M, Szarejko I (2015) Root hair development in the grasses: what we already know and what we still need to know. Plant Physiol 168:407–414
Meister R, Rajani MS, Ruzicka D, Schachtman DP (2014) Challenges of modifying root traits in crops for agriculture. Trends Plant Sci 19:779–788
Nomura T, Ishihara A, Yanagita RC, Endo TR, Iwamura H (2005) Three genomes differentially contribute to the biosynthesis of benzoxazinones in hexaploid wheat. Proc Natl Acad Sci USA 102:16490–16495
Osborn TC, Pires JC, Birchler JA, Auger DL, Chen ZJ, Lee HS, Comai L, Madlung A, Doerge RW, Colot V, Martienssen RA (2003) Understanding mechanisms of novel gene expression in polyploids. Trends Genet 19:141–147
Otto SP (2007) The evolutionary consequences of polyploidy. Cell 131:452–462
Peterson RL (1992) Adaptations of root structure in relation to biotic and abiotic factors. Can J Bot 70:661–675
Peterson RL, Farquhar ML (1996) Root hairs: specialized tubular cells extending root surfaces. Bot Rev 62:1–40
Shitsukawa N, Tahira C, Kassai K, Hirabayashi C, Shimizu T, Takumi S, Mochida K, Kawaura K, Ogihara Y, Murai K (2007) Genetic and epigenetic alteration among three homoeologous genes of a class E MADS box gene in hexaploid wheat. Plant Cell 19:1723–1737
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Willcox G (1998) Archaeobotanical evidence for the beginnings of agriculture in Southwest Asia. In: Damania AB, Valkoun J, Willcox G, Qualset CO (eds) The origins of agriculture and crop domestication. ICARDA, Aleppo, pp 25–38
Yang CW, Zhao L, Zhang HK, Yang ZZ, Wang H, Wen SS, Zhang CY, Rustgi S, von Wettstein D, Liu B (2014) Evolution of physiological responses to salt stress in hexaploid wheat. Proc Natl Acad Sci USA 111:11882–11887
Yi K, Menand B, Bell E, Dolan L (2010) A basic helix-loop-helix transcription factor controls cell growth and size in root hairs. Nat Genet 42:264–267
Zhang Z, Belcram H, Gornicki P, Charles M, Just J, Huneau C, Magdelenat G, Couloux A, Samain S, Gill BS, Rasmussen JB, Barbe V, Faris JD, Chalhoub B (2011) Duplication and partitioning in evolution and function of homoeologous Q loci governing domestication characters in polyploid wheat. Proc Natl Acad Sci USA 108:18737–18742
Acknowledgements
This research was supported by grants from the Major Program of the National Natural Science Foundation of China (31290212) and the National Key Research and Development Program of China (Grant No. 2016YFD0100801).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Han, H., Wang, H., Han, Y. et al. Altered expression of the TaRSL2 gene contributed to variation in root hair length during allopolyploid wheat evolution. Planta 246, 1019–1028 (2017). https://doi.org/10.1007/s00425-017-2735-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-017-2735-3