Abstract
Main conclusion
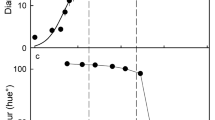
Xylem flow is progressively shut down during maturation beginning with minor veins at the stylar end and progressing to major veins and finally to bundles at the stem end.
This study investigates the functionality of the xylem vascular system in developing sweet cherry fruit (Prunus avium L.). The tracers acid fuchsin and gadoteric acid were fed to the pedicel of detached fruit. The tracer distribution was studied using light microscopy and magnetic resonance imaging. The vasculature of the sweet cherry comprises five major bundles. Three of these supply the flesh; two enter the pit to supply the ovules. All vascular bundles branch into major and minor veins that interconnect via numerous anastomoses. The flow in the xylem as indexed by the tracer distribution decreases continuously during development. The decrease is first evident at the stylar (distal) end of the fruit during pit hardening and progresses basipetally towards the pedicel (proximal) end of the fruit at maturity. That growth strains are the cause of the decreased conductance is indicated by: elastic strain relaxation after tissue excision, the presence of ruptured vessels in vivo, the presence of intrafascicular cavities, and the absence of tyloses.









Similar content being viewed by others
Abbreviations
- Gd DOTA:
-
Gadoteric acid
- MRI:
-
Magnetic resonance imaging
- RH:
-
Relative humidity
References
Bernstein Z, Lustig I (1981) A new method of firmness measurement of grape berries and other juicy fruit. Vitis 20:15–21
Bernstein Z, Lustig I (1985) Hydrostatic methods of measurement of firmness and turgor pressure of grape berries (Vitis vinifera L.). Sci Hortic 25:129–136
Bondada BR, Matthews MA, Shackel KA (2005) Functional xylem in the post-veraison grape berry. J Exp Bot 56:2949–2957
Brüggenwirth M, Knoche M (2015) Xylem conductance of sweet cherry pedicels. Trees 29:1851–1860. doi:10.1007/s00468-015-1266-4
Brüggenwirth M, Winkler A, Knoche M (2016) Xylem, phloem, and transpiration flows in developing sweet cherry fruit. Trees 30:1821–1830. doi:10.1007/s00468-016-1415-4
Bukovac MJ (1971) The nature and chemical promotion of abscission in maturing cherry fruit. HortScience 6:385–388
Chatelet DS, Rost TL, Shackel KA, Matthews MA (2008a) The peripheral xylem of grapevine (Vitis vinifera). 1. Structural integrity in post-veraison berries. J Exp Bot 59:1987–1996
Chatelet DS, Rost TL, Matthews MA, Shackel KA (2008b) The peripheral xylem of grapevine (Vitis vinifera) berries. 2. Anatomy and development. J Exp Bot 59:1997–2007
Choat B, Gambetta GA, Shackel KA, Matthews MA (2009) Vascular function in grape berries across development and its relevance to apparent hydraulic isolation. Plant Physiol 151:1677–1687
Christensen JV (1996) Rain-induced cracking of sweet cherries: Its causes and prevention. In: Webster AD, Looney NE (eds) Cherries: crop physiology, production and uses. CAB Intl, Wallingford, pp 297–327
Dean RJ, Stait-Gardener T, Clarke SJ, Rogiers SY, Bobek G, Price WS (2014) Use of diffusion magnetic resonance imaging to correlate the developmental changes in grape berry tissue structure with water diffusion pattern. Plant Methods 10:35. doi:10.1186/1746-4811-10-35
Dichio B, Remorini D, Lang S (2003) Developmental changes in xylem functionality in kiwifruit fruit: implications for fruit calcium accumulation. Acta Hortic 610:191–195
Dichio B, Montanaro G, Mazzeo M, Lang A (2011) Does dye infusion indicate xylem functionality in kiwifruit? Acta Hortic 913:353–355
Drazeta L, Lang A, Morgan L, Volz R, Jameson PE (2001) Bitter pit and vascular function in apples. Acta Hortic 564:387–392
Dražeta L, Lang A, Hall AJ, Volz RK, Jameson PE (2004) Causes and effects of changes in xylem functionality in apple fruit. Ann Bot 93:275–282
Düring H, Lang A, Oggionni F (1987) Patterns of water flow in Riesling berries in relation to developmental changes in their xylem morphology. Vitis 26:123–131
Findlay N, Oliver KJ, Nil N, Coombe BG (1987) Solute accumulation by grape pericarp cells. IV. Perfusion of pericarp apoplast via the pedicel and evidence for xylem malfunction in ripening berries. J Exp Bot 38:668–679
Geyer U, Schönherr J (1988) In vitro test for effects of surfactants and formulations on permeability of plant cuticles. In: Cross B, Scher HB (eds) Pesticide formulations: innovations and developments. American Chemical Society, Washington, pp 22–33
Grimm E, Peschel S, Becker T, Knoche M (2012) Stress and strain in the sweet cherry skin. J Am Soc Hortic Sci 137:383–390
Ho LC, Grange RI, Picken AJ (1987) An analysis of the accumulation of water and dry matter in tomato fruit. Plant Cell Environ 10:157–162
Karnovsky MJ (1965) A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron microscopy. J Cell Biol 27:137A–138A
Keller M, Smith JP, Bondada BR (2006) Ripening grape berries remain hydraulically connected to the shoot. J Exp Bot 57:2577–2587
Kenouche S, Perrier M, Bertin N, Larionova J, Ayadi A, Zanca M, Long J, Bezzi N, Stein PC, Guari Y, Cieslak M, Godin C, Goze-Bac C (2014) In vivo quantitative NMR imaging of fruit tissue during growth using spoiled gradient echo sequence. Magn Reson Imaging 32:1418–1427
Knipfer T, Fei J, Gambetta GA, McElrone AJ, Shackel KA, Matthews MA (2015) Water transport properties of the grape pedicel during fruit development: insights into xylem anatomy and function using microtomography. Plant Physiol 168:1590–1602
Knoche M, Peschel S, Hinz M, Bukovac MJ (2001) Studies on water transport through the sweet cherry surface: II. Conductance of the cuticle in relation to fruit development. Planta 213:927–936
Knoche M, Beyer M, Peschel S, Oparlakov B, Bukovac MJ (2004) Changes in strain and deposition of cuticle in developing sweet cherry fruit. Physiol Plant 120:667–677
Knoche M, Grimm E, Schlegel HJ (2014) Mature sweet cherries have low turgor. J Am Soc Hortic Sci 139:3–12
Kumar K, Sukumaran K, Taylor S, Chang AC, Nunn AD, Tweedle MF (1994) Partition coefficients (log P) and some capacity factors (k′) of some Gd(III) complexes of linear and macrocyclic polyamino carboxylates. J Liq Chromatogr 17:3735–3746
Lai X, Khanal BP, Knoche M (2016) Mismatch between cuticle deposition and area expansion in fruit skins allows potentially catastrophic buildup of elastic strain. Planta 244:1145–1156
Lang A (1990) Xylem, phloem and transpiration flows in developing apple fruits. J Exp Bot 41:645–651
Lang A, Düring H (1990) Grape berry splitting and some mechanical properties of the skin. Vitis 29:61–70
Lang A, Ryan KG (1994) Vascular development and sap flow in apple pedicels. Ann Bot 74:381–388
Lang A, Volz RK (1993) Leaf area, xylem cycling and Ca status in apples. Acta Hortic 343:89–92
Lang A, Volz RK (1998) Spur leaves increase calcium in young apples by promoting xylem inflow and outflow. J Am Soc Hortic Sci 123:956–960
Lindner U, Lingott J, Richter S, Jakubowski N, Panne U (2013) Speciation of gadolinium in surface water samples and plants by hydrophilic interaction chromatography hyphenated with inductively coupled plasma mass spectrometry. Anal Bioanal Chem 405:1865–1873
Mazzeo M, Dichio B, Clearwater MJ, Montanaro G, Xiloyannis C (2013) Hydraulic resistance of developing Actinidia fruit. Ann Bot 112:197–205
Morandi B, Manfrini L, Losciale P, Zibordi M, Grappadelli LC (2010) Changes in vascular and transpiration flows affect the seasonal and daily growth of kiwifruit (Actinidia deliciosa) berry. Ann Bot 105:913–923
Moriwaki S, Terada Y, Kose K, Haishi T, Sekozawa Y (2014) Visualization and quantification of vascular structure of fruit using magnetic resonance microimaging. Appl Magn Reson 45:517–525
Nordey T, Léchaudel M, Génard M (2015) The decline in xylem flow to mango fruit at the end of its development is related to the appearance of embolism in the fruit pedicel. Funct Plant Biol 42:668–675
Opara LU, Studman CJ, Banks NH (1997) Fruit skin splitting and cracking. Hortic Rev 19:217–262
Pope JM, Jonas D, Walker RR (1993) Applications of NMR micro-imaging to the study of water, lipid, and carbohydrate distribution in grape berries. Protoplasma 173:177–186
Rančić D, Quarrie SP, Radošević R, Terzić M, Pećinar I, Stikić R, Jansen S (2010) The application of various anatomical techniques for studying the hydraulic network in tomato fruit pedicels. Protoplasma 246:25–31
Redgwell RJ, MacRae E, Hallett I, Fischer M, Perry J, Harker R (1997) In vivo and in vitro swelling of cell walls during fruit ripening. Planta 203:162–173
Rogiers SY, Smith JA, White R, Keller M, Holzapfel BP, Virgona JM (2001) Vascular function in berries of Vitis vinifera (L) cv. Shiraz. Aust J Grape Wine Res 7:46–51
Schumann C, Schlegel HJ, Grimm E, Knoche M, Lang A (2014) Water potential and its components in developing sweet cherry. J Am Soc Hortic Sci 139:349–355
Sterling C (1953) Developmental anatomy of the fruit of Prunus domestica L. Bull Torrey Bot Club 80:457–477
Sterling C (1964) Comparative morphology of the carpel in the Rosaceae. I. Prunoidae: Prunus. Am J Bot 51:36–44
Stösser R, Rasmussen HP, Bukovac MJ (1969) A histological study of abscission layer formation in cherry fruits during maturation. J Am Soc Hortic Sci 94:239–243
Thomas TR, Matthews MA, Shackel KA (2006) Direct in situ measurement of cell turgor in grape (Vitis vinifera L.) berries during development and in response to plant water deficits. Plant Cell Environ 29:993–1001
Thomas TR, Shackel KA, Matthews MA (2008) Mesocarp cell turgor in Vitis vinifera L. berries throughout development and its relation to firmness, growth, and the onset of ripening. Planta 228:1067–1076
Tukey HB, Young JO (1939) Histological study of the developing fruit of the sour cherry. Bot Gaz 100:723–749
Van Dusschoten D, Metzner R, Kochs J, Postma JA, Pflugfelder D, Bühler J, Schurr U, Jahnke S (2016) Quantitative 3D analysis of plant roots growing in soil using magnetic resonance imaging. Plant Physiol 170:1176–1188
Winkler A, Brüggenwirth M, Ngo NS, Knoche M (2016) Fruit apoplast tension draws xylem water into mature sweet cherries. Sci Hortic 209:270–278
Wittenbach VA, Bukovac MJ (1972) An anatomical and histochemical study of abscission in maturing sweet cherry fruit. J Am Soc Hortic Sci 97:214–219
Acknowledgements
We thank Mr. Dieter Reese (Martin-Luther-University Halle-Wittenberg) for building the sample holder for MRI, and Dr. Alexander Lang (Sandy Lang Ltd, Eastbourne, NZ) and Dr. Siegfried Jahnke (Forschungszentrum Jülich, IBG-2, Germany) for thoughtful comments on this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grimm, E., Pflugfelder, D., van Dusschoten, D. et al. Physical rupture of the xylem in developing sweet cherry fruit causes progressive decline in xylem sap inflow rate. Planta 246, 659–672 (2017). https://doi.org/10.1007/s00425-017-2719-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-017-2719-3