Abstract
Main conclusion
Nitric oxide (NO)-mediated redox signaling plays a role in alleviating the negative impact of water stress in sugarcane plants by improving root growth and photosynthesis.
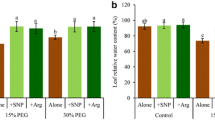
Drought is an environmental limitation affecting sugarcane growth and yield. The redox-active molecule nitric oxide (NO) is known to modulate plant responses to stressful conditions. NO may react with glutathione (GSH) to form S-nitrosoglutathione (GSNO), which is considered the main reservoir of NO in cells. Here, we investigate the role of NO in alleviating the effects of water deficit on growth and photosynthesis of sugarcane plants. Well-hydrated plants were compared to plants under drought and sprayed with mock (water) or GSNO at concentrations ranging from 10 to 1000 μM. Leaf GSNO sprayed plants showed significant improvement of relative water content and leaf and root dry matter under drought compared to mock-sprayed plants. Additionally, plants sprayed with GSNO (≥ 100 μM) showed higher leaf gas exchange and photochemical activity as compared to mock-sprayed plants under water deficit and after rehydration. Surprisingly, a raise in the total S-nitrosothiols content was observed in leaves sprayed with GSH or GSNO, suggesting a long-term role of NO-mediated responses to water deficit. Experiments with leaf discs fumigated with NO gas also suggested a role of NO in drought tolerance of sugarcane plants. Overall, our data indicate that the NO-mediated redox signaling plays a role in alleviating the negative effects of water stress in sugarcane plants by protecting the photosynthetic apparatus and improving shoot and root growth.





Similar content being viewed by others
Abbreviations
- A :
-
Leaf CO2 assimilation
- C i :
-
Intercellular CO2 concentration
- ETR:
-
Apparent electron transport rate
- F V/F M :
-
Maximum quantum efficiency of PSII
- g s :
-
Stomatal conductance
- GSH:
-
Glutathione
- GSNO:
-
S-Nitrosoglutathione
- k :
-
Instantaneous carboxylation efficiency
- LDM:
-
Leaf dry mass
- NO:
-
Nitric oxide
- NPQ:
-
Non-photochemical quenching
- PEG:
-
Polyethylene glycol
- PPFD:
-
Photosynthetic photon flux density
- PSII:
-
Photosystem II
- RDM:
-
Root dry mass
- RWC:
-
Relative water content
- RSNO:
-
S-Nitrosothiol
- WD:
-
Water deficit
- ϕ PSII :
-
Effective quantum efficiency of PSII
References
Aftab T, Khan MM, Naeem M, Idrees M, Moinuddin Teixeira, da Silva JA, Ram M (2012) Exogenous nitric oxide donor protects Artemisia annua from oxidative stress generated by boron and aluminum toxicity. Ecotox Environ Safe 80:60–68
Baker NR (2008) A probe of photosynthesis: in vivo. Annu Rev Plant Biol 59:89–113
Barbosa AM, Guidorizi KA, Catuchi TA, Marques TA, Ribeiro RV, Souza GM (2015) Biomass and bioenergy partitioning of sugarcane plants under water deficit. Acta Physiol Plant 37:137–142
Begara-Morales JC, Sánchez-Calvo B, Luque F, Leyva-Pérez MO, Leterrier M, Corpas FJ, Barroso JB (2014) Differential transcriptomic analysis by RNA-Seq of GSNO-responsive genes between Arabidopsis roots and leaves. Plant Cell Physiol 55:1080–1095
Besson-Bard A, Astier J, Rasul S, Wawer I, Dubreuil-Maurizi C, Jeandroz S, Wendehenne D (2009) Current view of nitric oxide-responsive genes in plants. Plant Sci 177:302–309
Bright J, Desikan R, Hancock JT, Weir IS, Neill SJ (2006) ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J 45:113–122
Cai W, Liu W, Wang WS, Fu ZW, Han TT, Lu YT (2015) Overexpression of rat neurons nitric oxide synthase in rice enhances drought and salt tolerance. PLoS One. doi:10.1371/journal.pone.0131599
Chaki M, Valderrama R, Fernández-Ocaña AM, Carreras A, Gómez-Rodríguez MV, López-Jaramillo J, Begara-Morales JC, Sánchez-Calvo B, Luque F, Leterrier M, Corpas FJ, Barroso JB (2011) High temperature triggers the metabolism of S-nitrosothiols in sunflower mediating a process of nitrosative stress which provokes the inhibition of ferredoxin–NADP reductase by tyrosine nitration. Plant Cell Environ 34:1803–1818
Correa-Aragunde N, Graziano M, Lamattina L (2004) Nitric oxide plays a central role in determining lateral root development in tomato. Planta 218:900–905
Correa-Aragunde N, Lanteri ML, García-Mata C, Have AT, Laxalt AM, Graziano M, Lamattina L (2007) Nitric oxide functions as intermediate in auxin, abscisic acid and lipid signaling pathways. In: Lamattina L, Polacco J (eds) Nitric oxide in plant growth, development and stress physiology, plant cell monographs, vol 5. Springer, Berlin, pp 113–130
Cruz de Carvalho MH (2008) Drought stress and reactive oxygen species: production, scavenging and signaling. Plant Signal Behav 3:156–165
De Oliveira MG, Shishido SM, Seabra AB, Morgon NH (2002) Thermal stability of primary S-nitrosothiols: roles of autocatalysis and structural effects on the rate of nitric oxide release. Phys Chem A 106:8963–8970
De Souza GFP, Yokoyama-Yasunaka JKU, Seabra AB, Miguel DC, de Oliveira MG, Uliana SRB (2006) Leishmanicidal activity of primary S-nitrosothiols against Leishmania major and Leishmania amazonensis: implications for the treatment of cutaneous leishmaniosis. Nitric Oxide 15:209–216
Edwards GE, Baker NR (1993) Can CO2 assimilation in maize leaves be predicted accurately from chlorophyll fluorescence analysis? Photosynth Res 37:89–102
Fang Y, Xiong L (2015) General mechanisms of drought response and their application in drought resistance improvement in plants. Cell Mol Life Sci 72:673–689
Farrant JM, Cooper K, Hilgart A, Abdalla KO, Bentley J, Thomson JA, Dace HJW, Peton N, Mundree SG, Rafudeen MS (2015) A molecular physiological review of vegetative desiccation tolerance in the resurrection plant Xerophyta viscosa (Baker). Planta 242:407–426
Foresi N, Mayta ML, Lodeyro AF, Scuffi D, Correa-Aragunde N, García-Mata C, Casalongué C, Carrillo N, Lamattina L (2015) Expression of the tetrahydrofolate-dependent nitric oxide synthase from the green alga Ostreococcus tauri increases tolerance to abiotic stresses and influences stomatal development in Arabidopsis. Plant J 82:806–821
Frungillo L, De Oliveira JF, Saviani EE, Oliveira HC, Martínez MC, Salgado I (2013) Modulation of mitochondrial activity by S-nitrosoglutathione reductase in Arabidopsis thaliana transgenic cell lines. Biochim Biophys Acta 1827:239–247
Frungillo L, Skelly MJ, Loake GJ, Spoel SH, Salgado I (2014) S-Nitrosothiols regulate nitric oxide production and storage in plants through the nitrogen assimilation pathway. Nat Commun 5:5401–5410
García-Mata CG, Lamattina L (2001) Nitric oxide induces stomatal closure and enhances the adaptive plant responses against drought stress. Plant Physiol 126:1196–1204
Gouvea CMCP, Souza JF, Magalhães ACN, Martins IS (1997) NO-releasing substances that induce growth elongation in maize root segments. Plant Growth Regul 21:183–187
Jamaux I, Steinmetz A, Belhassen E (1997) Looking for molecular and physiological markers of osmotic adjustment in sunflower. New Phytol 137:117–127
Kato H, Takemoto D, Kawakita K (2013) Proteomic analysis of S-nitrosylated proteins in potato plant. Physiol Plant 148:371–386
Kneeshaw S, Gelineau S, Tada Y, Loake GJ, Spoel SH (2014) Selective protein denitrosylation activity of thioredoxin-h5 modulates plant immunity. Mol Cell 56:153–162
Lee KH, Piao HL, Kim HY, Choi SM, Jiang F, Hartung W, Hwang I, Kwak JM, Lee IJ, Hwang I (2006) Activation of glucosidase via stress-induced polymerization rapidly increases active pools of abscisic acid. Cell 126:1109–1120
Lindermayr C, Saalbach G, Durner J (2005) Proteomic identification of S-nitrosylated proteins in Arabidopsis. Plant Physiol 137:921–930
Liu L, Hausladen A, Zeng M, Que L, Heitman J, Stamler JS (2001) A metabolic enzyme for S-nitrosothiol conserved from bacteria to humans. Nature 410:490–494
Long SP, Bernacchi CJ (2003) Gas exchange measurements, what can they tell us about the underlying limitations to photosynthesis? Procedures and sources of error. J Exp Bot 4:2393–2401
Machado RS, Ribeiro RV, Marchiori PER, Machado DFSP, Machado EC, Landell MGA (2009) Respostas biométricas e fisiológicas ao déficit hídrico em cana-de-açúcar em diferentes fases fenológicas. Pesq Agropec Bras 44:1575–1582
Misra NA, Vladkova R, Singh R, Misra M, Dobrikova AG, Apostolova EL (2014) Action and target sites of nitric oxide in chloroplasts. Nitric Oxide 39:35–45
Ördög A, Wodala B, Rózsavölgyi T, Tari Irma, Horváth F (2013) Regulation of guard cell photosynthetic electron transport by nitric oxide. J Exp Bot 64:1357–1366
Pagnussat GC, Simontacchi M, Puntarulo S, Lamattina L (2002) Nitric oxide is required for root organogenesis. Plant Physiol 129:954–956
Parry MAJ, Habash D, Araus JL (2004) Optimisation of water use by plants. Ann Appl Biol 144:125–126
Radi R (2004) Nitric oxide, oxidants, and protein tyrosine nitration. PNAS 101:4003–4008
Ramesh P (2000) Effect of different levels of drought during the formative phase on growth parameters and its relationship with dry matter accumulation in sugarcane. J Agron Crop Sci 85:83–89
Ribeiro RV, Machado RS, Machado EC, Machado DFSP, Magalhães Filho JR, Landell MGA (2013) Revealing drought-resistance and productive patterns in sugarcane genotypes by evaluating both physiological responses and stalk yield. Exp Agric 49:212–224
Sales CRG, Ribeiro RV, Silveira JAG, Machado EC, Martins OM, Lagôa AMMA (2013) Superoxide dismutase and ascorbate peroxidase improve the recovery of photosynthesis in sugarcane plants subjected to water deficit and low substrate temperature. Plant Physiol Biochem 73:326–336
Sales CRG, Marchiori PER, Machado RS, Fontenele AV, Machado EC, Silveira JAG, Ribeiro RV (2015) Photosynthetic and antioxidant responses to drought during the sugarcane ripening. Photosynthetica 53:547–554
Salgado I, Martínez MC, Oliveira HC, Frungillo L (2013) Nitric oxide signaling and homeostasis in plants: a focus on nitrate reductase and S-nitrosoglutathione reductase in stress-related responses. Braz J Bot 36:89–98
Santisree P, Bhatnagar-Mathur P, Sharma KK (2015) NO to drought-multifunctional role of nitric oxide in plant drought: do we have all the answers? Plant Sci 239:44–55
Santos MC, Seabra AB, Pelegrino MT, Haddad PS (2016) Synthesis, characterization and cytotoxicity of glutathione- and PEG-glutathione-superparamagnetic iron oxide nanoparticles for nitric oxide delivery. Appl Surf Sci 367:26–35
Santos-Filho PR, Vitor SC, Frungillo L, Saviani EE, Oliveira HC, Salgado I (2012) Nitrate reductase and nitric oxide-dependent activation of sinapoylglucose: malate sinapoyltransferase in leaves of Arabidopsis thaliana. Plant Cell Physiol 53:1607–1616
Sanz L, Fernández-Marcos M, Modrego A, Lewis DR, Muday GK, Pollmann S, Dueñas M, Santos-Buelga C, Lorenzo O (2014) Nitric oxide plays a role in stem cell niche homeostasis through its interaction with auxin. Plant Physiol 166:1972–1984
Sarruge JR (1975) Soluções nutritivas. Summa Phytopathol 3:231–233
Seabra AB, de Oliveira MG (2004) Poly (vinyl alcohol) and poly (vinyl pyrrolidone) blended films for local nitric oxide release. Biomaterials 25:3773–3782
Sharp RE (2002) Interaction with ethylene: changing views on the role of abscisic acid in root and shoot growth responses to water stress. Plant Cell Environ 25:211–222
Sharp RE, LeNoble ME (2002) ABA, ethylene and the control of shoot and root growth under water stress. J Exp Bot 53:33–37
Shishido SM, Seabra AB, Loh W, De Oliveira MG (2003) Thermal and photochemical nitric oxide release from S-nitrosothiols incorporated in Pluronic F127gel: potential uses for local and controlled nitric oxide release. Biomaterials 24:3543–3553
Simontacchi M, Galatro A, Ramos-Artuso F, Santa-María GE (2015) Plant survival in a changing environment: the role of nitric oxide in plant responses to abiotic stress. Front Plant Sci 6:977–996
Spadaro D, Yun BW, Spoel SH, Chu C, Wang YQ, Loake GJ (2010) The redox switch: dynamic regulation of protein function by cysteine modifications. Physiol Plant 138:360–371
Stamler JS, Singel DJ, Loscalzo J (1992) Biochemistry of nitric oxide and its redox-activated forms. Science 258:1898–1902
Takahashi S, Yamasaki H (2002) Reversible inhibition of photophosphorylation in chloroplasts by nitric oxide. FEBS Lett 512:145–148
Tian X, Lei Y (2006) Nitric oxide treatment alleviates drought stress in wheat seedlings. Biol Plant 50:775–778
Vitor SC, Duarte GT, Saviani EE, Vincentz MGA, Oliveira HC, Salgado I (2013) Nitrate reductase is required for the transcriptional modulation and bactericidal activity of nitric oxide during the defense response of Arabidopsis thaliana against Pseudomonas syringae. Planta 238:475–486
Wang P, Du Y, Hou YJ, Zhao Y, Hsu CC, Yuan F, Zhu X, Tao WA, Song CP, Zhu JK (2015) Nitric oxide negatively regulates abscisic acid signaling in guard cells by S-nitrosylation of OST1. Proc Natl Acad Sci USA 112:613–618
Wodala B, Deák Z, Vass I, Erdei L, Altorjay I, Horváth F (2008) In vivo target sites of nitric oxide in photosynthetic electron transport as studied by chlorophyll fluorescence in pea leaves. Plant Physiol 146:1920–1927
Yang LT, Qi YP, Chen LS, Sang W, Lin XJ, Wu YL, Yang CJ (2012) Nitric oxide protects sour pummelo (Citrus grandis) seedlings against aluminum-induced inhibition of growth and photosynthesis. Environ Exp Bot 82:1–13
Yu M, Lamattina L, Spoel SH, Loake GJ (2014) Nitric oxide function in plant biology: a redox cue in deconvolution. New Phytol 202:1142–1156
Yun BW, Feechan A, Yin M, Saidi NBB, Bihan TL, Yu M, Moore JW, Kang JG, Kwon E, Spoel SH, Pallas JA, Loake GJ (2011) S-Nitrosylation of NADPH oxidase regulates cell death in plant immunity. Nature 478:264–268
Zhang X, Cardosa L, Broderick M, Fein H, Davies IR (2000) Novel calibration method for nitric oxide microsensors by stoichiometrical generation of nitric oxide from SNAP. Electroanal 12:425–428
Acknowledgments
The authors acknowledge the financial support (BIOEN Program, Grant no. 2008/57519-2) provided by the São Paulo Research Foundation (FAPESP, Brazil) as well as the scholarships to NMS and MTP (Grant no. 2012/19167-0 and 2015/00393-8). LF is a European Molecular Biology Organization (EMBO)—Long Term Fellow (no. 420/2015). The authors also acknowledge the fellowships (ABS; IS; ECM; RVR) and scholarships (FCCM and MTM) granted by the National Council for Scientific and Technological Development (CNPq, Brazil).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Silveira, N.M., Frungillo, L., Marcos, F.C.C. et al. Exogenous nitric oxide improves sugarcane growth and photosynthesis under water deficit. Planta 244, 181–190 (2016). https://doi.org/10.1007/s00425-016-2501-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-016-2501-y