Abstract
Main conclusion
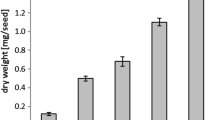
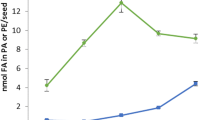
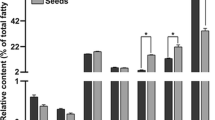
Plant acylcarnitines are present during anabolic processes of lipid metabolism. Their low contents relatively to the corresponding acyl-CoAs suggest that they are associated to specific pools of activated fatty acids.
The non-proteinaceous amino acid carnitine exists in plants either as a free form or esterified to fatty acids. To clarify the biological significance of acylcarnitines in plant lipid metabolism, we have analyzed their content in plant extracts using an optimized tandem mass spectrometry coupled to liquid chromatography method. We have studied different developmental processes (post-germination, organogenesis, embryogenesis) targeted for their high requirement for lipid metabolism. The modulation of the acylcarnitine content was compared to that of the lipid composition and lipid biosynthetic gene expression level in the analyzed materials. Arabidopsis mutants were also studied based on their alteration in de novo fatty acid partitioning between the prokaryotic and eukaryotic pathways of lipid biosynthesis. We show that acylcarnitines cannot specifically be associated to triacylglycerol catabolism but that they are also associated to anabolic pathways of lipid metabolism. They are present during membrane and storage lipid biosynthesis processes. A great divergence in the relative contents of acylcarnitines as compared to the corresponding acyl-CoAs suggests that acylcarnitines are associated to very specific process(es) of lipid metabolism. The nature of their involvement as the transport form of activated fatty acids or in connection with the management of acyl-CoA pools is discussed. Also, the occurrence of medium-chain entities suggests that acylcarnitines are associated with additional lipid processes such as protein acylation for instance. This work strengthens the understanding of the role of acylcarnitines in plant lipid metabolism, probably in the management of specific acyl-CoA pools.







Similar content being viewed by others
Abbreviations
- ACP:
-
Acyl carrier protein
- CAT:
-
Carnitine acetyltransferase
- CPT:
-
Carnitine palmitoyltransferase
- FA:
-
Fatty acid
- FAT:
-
Fatty acyl-ACP thioesterase
- FFA:
-
Free fatty acid
- KAS:
-
3-Ketoacyl-ACP synthase
- LACS:
-
Long-chain acyl-CoA synthase
- LPAAT:
-
Lysophosphatidic acid acyltransferase
- LPCAT:
-
Lysophosphatidylcholine acyltransferase
- RF:
-
Response factor
- TAG:
-
Triacylglycerol
- WT:
-
Wild type
References
Abo-Hashema KA, Cake MH, Power GW, Clarke D (1999) Evidence for triacylglycerol synthesis in the lumen of microsomes via a lipolysis-esterification pathway involving carnitine acyltransferases. J Biol Chem 274:35577–35582
Antonenkov VD, Hiltunen JK (2012) Transfer of metabolites across the peroxisomal membrane. Biochim Biophys Acta 1822:1374–1386
Bach L, Gissot L, Marion J, Tellier F, Moreau P, Satiat-Jeunemaître B, Palauqui JC, Napier JA, Faure JD (2011) Very-long-chain fatty acids are required for cell plate formation during cytokinesis in Arabidopsis thaliana. J Cell Sci 124:3223–3234
Bates PD, Stymne S, Ohlrogge J (2013) Biochemical pathways in seed oil synthesis. Curr Opin Plant Biol 16:358–364
Benning C (2009) Mechanisms of lipid transport involved in organelle biogenesis in plant cells. Annu Rev Cell Dev Biol 25:71–91
Bonaventure G, Salas JJ, Pollard MR, Ohlrogge JB (2003) Disruption of the FATB gene in Arabidopsis demonstrates an essential role of saturated fatty acids in plant growth. Plant Cell 15:1020–1033
Bonaventure G, Bao X, Ohlrogge J, Pollard M (2004) Metabolic responses to the reduction in palmitate caused by disruption of the FATB gene in Arabidopsis. Plant Physiol 135:1269–1279
Bourdin B, Adenier H, Perrin Y (2007) Carnitine is associated with fatty acid metabolism in plants. Plant Physiol Biochem 45:926–931
Burgess N, Thomas DR (1986) Carnitine acyltransferase in pea cotyledon mitochondria. Planta 167:58–65
Cruz-Ramírez A, López-Bucio J, Ramírez-Pimentel G, Zurita-Silva A, Sánchez-Calderon L, Ramírez-Chávez E, González-Ortega E, Herrera-Estrella LL (2004) The xipotl mutant of Arabidopsis reveals a critical role for phospholipid metabolism in root system development and epidermal cell integrity. Plant Cell 16:2020–2034
Elgersma Y, van Roermund CW, Wanders RJ, Tabak HF (1995) Peroxisomal and mitochondrial carnitine acetyltransferases of Saccharomyces cerevisiae are encoded by a single gene. EMBO J 14:3472–3479
Ewald R, Hoffmann C, Florian A, Neuhaus E, Fernie AR, Bauwe H (2014) Lipoate-protein ligase and octanoyltransferase are essential for protein lipoylation in mitochondria of Arabidopsis. Plant Physiol 165:978–990
Footitt S, Slocombe SP, Larner V, Kurup S, Wu Y, Larson T, Graham I, Baker A, Holdsworth M (2002) Control of germination and lipid mobilization by COMATOSE, the Arabidopsis homologue of human ALDP. EMBO J 21:2912–2922
Fraenkel G (1953) Studies on the distribution of vitamin BT (carnitine). Biol Bull 104:359–371
Fraser F, Zammit VA (1999) Submitochondrial and subcellular distributions of the carnitine-acylcarnitine carrier. FEBS Lett 445:41–44
Gerbling H, Gerhardt B (1988) Carnitine-acyltransferase activity of mitochondria from mung-bean hypocotyls. Planta 174:90–93
Gooding JM, Shayeghi M, Saggerson ED (2004) Membrane transport of fatty acylcarnitine and free l-carnitine by rat liver microsomes. Eur J Biochem 271:954–961
Graham IA, Eastmond PJ (2002) Pathways of straight and branched chain fatty acid catabolism in higher plants. Prog Lipid Res 41:156–181
Gutierrez L, Mongelard G, Floková K, Pacurar DI, Novák O, Staswick P, Kowalczyk M, Pacurar M, Demailly H, Geiss G, Bellini C (2012) Auxin controls Arabidopsis adventitious root initiation by regulating jasmonic acid homeostasis. Plant Cell 24:2515–2527
Kim S, Yamaoka Y, Ono H, Kim H, Shim D, Maeshima M, Martinoia E, Cahoon EB, Nishida I, Lee Y (2013) AtABCA9 transporter supplies fatty acids for lipid synthesis to the endoplasmic reticulum. Proc Natl Acad Sci USA 110:773–778
Koo AJ, Ohlrogge JB, Pollard M (2004) On the export of fatty acids from the chloroplast. J Biol Chem 279:16101–16110
Kunst L, Browse J, Somerville C (1988) Altered regulation of lipid biosynthesis in a mutant of Arabidopsis deficient in chloroplast glycerol-3-phosphate acyltransferase activity. Proc Natl Acad Sci USA 85:4143–4147
Li N, Gügel IL, Giavalisco P, Zeisler V, Schreiber L, Soll J, Philippar K (2015) FAX1, a novel membrane protein mediating plastid fatty acid export. PLoS Biol 13:e1002053
Li-Beisson Y, Shorrosh B, Beisson F, Andersson MX, Arondel V, Bates PD, Baud S, Bird D, Debono A, Durrett TP, Franke RB, Graham IA, Katayama K, Kelly AA, Larson T, Markham JE, Miquel M, Molina I, Nishida I, Rowland O, Samuels L, Schmid KM, Wada H, Welti R, Xu C, Zallot R, Ohlrogge J (2013) Acyl-lipid metabolism. Arabidopsis Book 11:e0161
Masterson C, Wood C (2000) Pea chloroplast carnitine acetyltransferase. Proc Biol Sci 267:1–6
Masterson C, Wood C (2009) Influence of mitochondrial beta-oxidation on early pea seedling development. New Phytol 181:832–842
McLaren I, Wood C, Jalil MNH, Yong BCS, Thomas DR (1985) Carnitine acyltransferases in chloroplasts of Pisum sativum L. Planta 163:197–200
McNeil PH, Thomas DR (1975) Carnitine content of pea seedling cotyledons. Phytochemistry 14:2335–2336
McNeil PH, Thomas DR (1976) The effect of carnitine on palmitate oxidation by pea cotyledon mitochondria. J Exp Bot 27:1163–1179
Nameth B, Dinka SJ, Chatfield SP, Morris A, English J, Lewis D, Oro R, Raizada MN (2013) The shoot regeneration capacity of excised Arabidopsis cotyledons is established during the initial hours after injury and is modulated by a complex genetic network of light signaling. Plant, Cell Environ 36:68–86
Ohlrogge J, Browse J (1995) Lipid biosynthesis. Plant Cell 7:957–970
Panter RA, Mudd JB (1969) Carnitine levels in some higher plants. FEBS Lett 5:169–170
Ramsay RR, Zammit VA (2004) Carnitine acyltransferases and their influence on CoA pools in health and disease. Mol Aspects Med 25:475–493
Ramsay RR, Gandour RD, Van Der Leij FR (2001) Molecular enzymology of carnitine transfer and transport. Biochem Biophys Acta Protein Struct Mol Enzymol 1546:21–43
Running MP (2014) The role of lipid post-translational modification in plant developmental processes. Front Plant Sci 5:50
Salas JJ, Ohlrogge JB (2002) Characterization of substrate specificity of plant FatA and FatB acyl-ACP thioesterases. Arch Biochem Biophys 403:25–34
Schwabedissen-Gerbling H, Gerhardt B (1995) Purification and characterization of carnitine acyltransferase from higher plant mitochondria. Phytochemistry 39:36–43
Shockey JM, Fulda MS, Browse J (2002) Arabidopsis contains nine long-chain acyl-coenzyme a synthetase genes that participate in fatty acid and glycerolipid metabolism. Plant Physiol 129:1710–1722
Sierra AY, Gratacós E, Carrasco P, Clotet J, Ureña J, Serra D, Asins G, Hegardt FG, Casals N (2008) CPT1c is localized in endoplasmic reticulum of neurons and has carnitine palmitoyltransferase activity. J Biol Chem 283:6878–6885
Steiber A, Kerner J, Hoppel CL (2004) Carnitine: a nutritional, biosynthetic, and functional perspective. Mol Aspects Med 25:455–473
Stephens FB, Constantin-Teodosiu D, Greenhaff PL (2007) New insights concerning the role of carnitine in the regulation of fuel metabolism in skeletal muscle. J Physiol 581:431–444
ter Veld F, Primassin S, Hoffmann L, Mayatepek E, Spiekerkoetter U (2009) Corresponding increase in long-chain acyl-CoA and acylcarnitine after exercise in muscle from VLCAD mice. J Lipid Res 50:1556–1562
Thomas DR, Wood C (1986) The two β-oxidation sites in pea cotyledons carnitine palmitoyltransferase: location and function in pea mitochondria. Planta 168:261–266
Tjellström H, Yang Z, Allen DK, Ohlrogge JB (2012) Rapid kinetic labeling of Arabidopsis cell suspension cultures: implications for models of lipid export from plastids. Plant Physiol 158:601–611
van Roermund CW, Waterham HR, Ijlst L, Wanders RJ (2003) Fatty acid metabolism in Saccharomyces cerevisiae. Cell Mol Life Sci 60:1838–1851
Vrkoslav V, Cvačka J (2012) Identification of the double-bond position in fatty acid methyl esters by liquid chromatography/atmospheric pressure chemical ionisation mass spectrometry. J Chromatogr A 1259:244–250
Wang Z, Benning C (2012) Chloroplast lipid synthesis and lipid trafficking through ER-plastid membrane contact sites. Biochem Soc Trans 40:457–463
Washington L, Cook GA, Mansbach CM 2nd (2003) Inhibition of carnitine palmitoyltransferase in the rat small intestine reduces export of triacylglycerol into the lymph. J Lipid Res 44:1395–1403
Wood C, Jalil MNH, Ariffin A, Yong BCS, Thomas DR (1983) Carnitine short-chain acyltransferases in pea mitochondria. Planta 158:175–178
Wood C, Jalil MNH, McLaren I, Yong BCS, Ariffin A, McNeil PH, Burgess N, Thomas DR (1984) Carnitine long-chain acyltransferase and oxidation of palmitate, palmitoyl CoA and palmitoylcarnitine by pea mitochondria preparations. Planta 161:255–260
Zammit VA, Ramsay RR, Bonomini M, Arduini A (2009) Carnitine, mitochondrial function and therapy. Adv Drug Deliv Rev 61:1353–1362
Zhao L, Katavic V, Li F, Haughn GW, Kunst L (2010) Insertional mutant analysis reveals that long-chain acyl-CoA synthetase 1 (LACS1), but not LACS8, functionally overlaps with LACS9 in Arabidopsis seed oil biosynthesis. Plant J 64:1048–1058
Acknowledgments
We are grateful to Dr. Laurent Gutierrez and Dr. Stéphanie Guénin from Université de Picardie Jules Verne (Amiens, France) for their assistance in RT-qPCR analyses and Franck Merlier from our group for his technical support in mass spectrometry analysis. We are grateful to Dr. Gustavo Bonaventure from Max Planck Institute (Jena, Germany) for kindly providing with the Arabidopsis mutant genotypes. We also thank a lot Dr. George Lomonossoff from John Innes Centre (Norwich, UK) for taking time to carefully read the manuscript. Finally, we appreciate the time and helpful comments from the reviewers. The work was supported by the French Ministry of National Education, Higher Education and Research.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nguyen, PJ., Rippa, S., Rossez, Y. et al. Acylcarnitines participate in developmental processes associated to lipid metabolism in plants. Planta 243, 1011–1022 (2016). https://doi.org/10.1007/s00425-016-2465-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-016-2465-y