Abstract
Main conclusion
A RhoA-derived peptide fused to carrier molecules from plants showed enhanced biological activity of in vitro assays against respiratory syncytial virus compared to the RhoA peptide alone or the synthetic RhoA peptide.
A RhoA-derived peptide has been reported for over a decade as a potential inhibitor of respiratory syncytial virus (RSV) infection both in vitro and in vivo and is anticipated to be a promising alternative to monoclonal antibody-based therapy against RSV infection. However, there are several challenges to furthering development of this antiviral peptide, including improvement in the peptide’s bioavailability, development of an efficient delivery system and identification of a cost-effective production platform. In this study, we have engineered a RhoA peptide as a genetic fusion to two carrier molecules, either lichenase (LicKM) or the coat protein (CP) of Alfalfa mosaic virus. These constructs were introduced into Nicotiana benthamiana plants using a tobacco mosaic virus-based expression vector and targets purified. The results demonstrated that the RhoA peptide fusion proteins were efficiently expressed in N. benthamiana plants, and that two of the resulting fusion proteins, RhoA-LicKM and RhoA2-FL-d25CP, inhibited RSV growth in vitro by 50 and 80 %, respectively. These data indicate the feasibility of transient expression of this biologically active antiviral RhoA peptide in plants and the advantage of using a carrier molecule to enhance target expression and efficacy.






Similar content being viewed by others
References
Budge PJ, Lebowitz J, Graham BS (2003) Antiviral activity of RhoA-derived peptides against respiratory syncytial virus is dependent on formation of peptide dimers. Antimicrob Agents Chemother 47:3470–3477
Budge PJ, Li Y, Beeler JA, Graham BS (2004) RhoA-derived peptide dimers share mechanistic properties with other polyanionic inhibitors of respiratory syncytial virus (RSV), including disruption of viral attachment and dependence on RSV G. J Virol 78:5015–5022
Buttery JP, Moxon ER (2000) Designing meningitis vaccines. J R Coll Phys Lond 34:163–168
Chichester JA, Musiychuk K, de la Rosa P, Horsey A, Stevenson N, Ugulava N, Rabindran S, Palmer GA, Mett V, Yusibov V (2007) Immunogenicity of a subunit vaccine against Bacillus anthracis. Vaccine 25:3111–3114
Collins PL, McIntosh K, Chanock RM (1996) Respiratory syncytial virus. In: Fields BN (ed) Fields virology. Raven Press, New York, pp 1313–1351
Cummings JF, Guerrero ML, Moon JE, Waterman P, Nielsen RK, Jefferson S, Gross FL, Hancock K, Katz JM, Yusibov V, Fraunhofer USA Center for Molecular Biotechnology Study Group (2014) Safety and immunogenicity of a plant-produced recombinant monomer hemagglutinin-based influenza vaccine derived from influenza A (H1N1)pdm09 virus: a Phase 1 dose-escalation study in healthy adults. Vaccine 32:2251–2259. doi:10.1016/j.vaccine.2013.10.017
DeVincenzo JP, Aitken J, Harrison L (2003) Respiratory syncytial virus (RSV) loads in premature infants with and without prophylactic RSV fusion protein monoclonal antibody. J Pediatr 143:123–126
Douglas JL, Panis ML, Ho E, Lin KY, Krawczyk SH, Grant DM, Cai R, Swaminathan S, Cihlar T (2003) Inhibition of respiratory syncytial virus fusion by the small molecule VP-14637 via specific interactions with F protein. J Virol 77:5054–5064
Giddings G (2001) Transgenic plants as protein factories. Curr Opin Biotechnol 12:450–454
Gleba Y, Klimyuk V, Marillonnet S (2007) Viral vectors for the expression of proteins in plants. Curr Opin Biotechnol 18:134–141
Green BJ, Fujiki M, Mett V, Kaczmarczyk J, Shamloul M, Musiychuk K, Underkoffler S, Yusibov V, Mett V (2009) Transient protein expression in three Pisum sativum (green pea) varieties. Biotechnol J 4:230–237. doi:10.1002/biot.200800256
Hellens RP, Edwards EA, Leyland NR, Bean S, Mullineaux PM (2000) pGreen: a versatile and flexible binary Ti vector for Agrobacterium-mediated plant transformation. Plant Mol Biol 42:819–832
Hood EE, Kusnadi A, Nikolov Z, Howard JA (1999) Molecular farming of industrial proteins from transgenic maize. Adv Exp Med Biol 464:127–147
Jacobson RM, Poland GA (2002) The pneumococcal conjugate vaccine. Minerva Pediatr 54:295–303
Jones RM, Chichester JA, Mett V, Jaje J, Tottey S, Manceva S, Casta LJ, Gibbs SK, Musiychuk K, Shamloul M, Norikane J, Mett V, Streatfield SJ, van de Vegte-Bolmer M, Roeffen W, Sauerwein RW, Yusibov V (2013) A plant-produced Pfs25 VLP malaria vaccine candidate induces persistent transmission blocking antibodies against Plasmodium falciparum in immunized mice. PLoS One 8:e79538. doi:10.1371/journal.pone.0079538
Kapikian AZ, Mitchell RH, Chanock RM, Shvedoff RA, Stewart CE (1969) An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am J Epidemiol 89:405–421
Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, Parrott RH (1969) Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol 89:422–434
Malley R, De Vincenzo J, Ramilo O, Dennehy PH, Meissner HC, Gruber WC, Sanchez PJ, Jafri H, Balsley J, Carlin D, Buckingham S, Vernacchio L, Ambrosino DM (1998) Reduction of respiratory syncytial virus (RSV) in tracheal aspirates in intubated infants by use of humanized monoclonal antibody to RSV F protein. J Infect Dis 178:1555–1561
Maselko M, Ward C, Pastey M (2011) A RhoA-derived peptide inhibits human immunodeficiency virus-1 entry in vitro. Curr HIV Res 9:1–5
Mett V, Lyons J, Musiychuk K, Chichester JA, Brasil T, Couch R, Sherwood R, Palmer GA, Streatfield SJ, Yusibov V (2007) A plant-produced plague vaccine candidate confers protection to monkeys. Vaccine 25:3014–3017
Montvale, NJ (2010) Red book: pharmacy’s fundamental reference. Thomson PDR
Musiychuk K, Stephenson N, Bi H, Farrance CE, Orozovic G, Brodelius M, Brodelius P, Horsey A, Ugulava N, Shamloul AM, Mett V, Rabindran S, Streatfield SJ, Yusibov V (2007) A launch vector for the production of vaccine antigens in plants. Influenza Other Respir Viruses 1:19–25. doi:10.1111/j.17502659.2006.00005.x
Narumiya S (1996) The small GTPase Rho: cellular functions and signal transduction. J Biochem 120:215–228
Pastey MK, Crowe JE Jr, Graham BS (1999) RhoA interacts with the fusion glycoprotein of respiratory syncytial virus and facilitates virus-induced syncytium formation. J Virol 73:7262–7270
Pastey MK, Gower TL, Spearman PW, Crowe JE Jr, Graham BS (2000) A RhoA-derived peptide inhibits syncytium formation induced by respiratory syncytial virus and parainfluenza virus type 3. Nat Med 6:35–40
Rybicki EP (2010) Plant-made vaccines for humans and animals. Plant Biotechnol J 8:620–637. doi:10.1111/j.1467-7652.2010.00507.x
Schillberg S, Twyman RM, Fischer R (2005) Opportunities for recombinant antigen and antibody expression in transgenic plants-technology assessment. Vaccine 23:1764–1769
Sharma AK, Sharma MK (2009) Plants as bioreactors: recent developments and emerging opportunities. Biotechnol Adv 27:811–832
Shoji Y, Chichester JA, Bi H, Musiychuk K, de la Rosa P, Goldschmidt L, Horsey A, Ugulava N, Palmer GA, Mett V, Yusibov V (2008) Plant-expressed HA as a seasonal influenza vaccine candidate. Vaccine 26:2930–2934. doi:10.1016/j.vaccine.2008.03.045
Shoji Y, Chichester JA, Jones M, Manceva SD, Damon E, Mett V, Musiychuk K, Bi H, Farrance C, Shamloul M, Kushnir N, Sharma S, Yusibov V (2011) Plant-based rapid production of recombinant subunit hemagglutinin vaccines targeting H1N1 and H5N1 influenza. Hum. Vaccin. 7:41–45
Stein RT, Sherrill D, Morgan WJ, Holberg CJ, Halonen M, Taussig LM, Wright AL, Martinez FD (1999) Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet 354:541–545
Tiwari S, Verma PC, Singh PK, Tuli R (2009) Plants as bioreactors for the production of vaccine antigens. Biotechnol Adv 27:449–467. doi:10.1016/j.biotechadv.2009.03.006
Tran DN, Pham TM, Ha MT, Tran TT, Dang TK, Yoshida LM, Okitsu S, Hayakawa S, Mizuguchi M, Ushijima H (2013) Molecular epidemiology and disease severity of human respiratory syncytial virus in Vietnam. PLoS One 8(1):e45436. doi:10.1371/journal.pone.0045436
Turner TL, Kopp BT, Paul G, Landgrave LC, Hayes D Jr, Thompson R (2014) Respiratory syncytial virus: current and emerging treatment options. Clinicoecon Outcom Res 6:217–225. doi:10.2147/CEOR.S60710
Vella PP, Ellis RW (1992) Haemophilus b conjugate vaccines. Biotechnology 20:1–22
Vitale A, Pedrazzini E (2005) Recombinant pharmaceuticals from plants: the plant endomembrane system as bioreactor. Mol Interv 5:216–225
Yin J, Li G, Ren X, Herrler G (2007) Select what you need: a comparative evaluation of the advantages and limitations of frequently used expression systems for foreign genes. J Biotechnol 127:335–347
Yu KL, Zhang Y, Civiello RL, Kadow KF, Cianci C, Krystal M, Meanwell NA (2003) Fundamental structure-activity relationships associated with a new structural class of respiratory syncytial virus inhibitor. Bioorg Med Chem Lett 13:2141–2144
Yusibov V, Hooper DC, Spitsin SV, Fleysh N, Kean RB, Mikheeva T, Deka D, Karasev A, Cox S, Randall J, Koprowski H (2002) Expression in plants and immunogenicity of plant virus-based experimental rabies vaccine. Vaccine 20:3155–3164
Yusibov V, Streatfield SJ, Kushnir N, Roy G, Padmanaban A (2013) Hybrid viral vectors for vaccine and antibody production in plants. Curr Pharm Des 19:5574–5586
Acknowledgments
The authors are grateful to Dr. Natasha Kushnir for editorial assistance. We also thank CONACyT Mexico for the grant 154790 and scholarship 233531, granted to Ortega-Berlanga for her PhD studies
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
425_2015_2416_MOESM1_ESM.tif
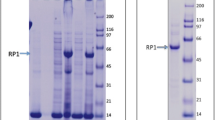
Figure 1S. SDS-PAGE of LickM-fusion and CP-fusion proteins after purification. (A) Coomassie gel of LickM fusion protein (PR-RhoA2-FL-F-3-HK). M: Molecular marker (See blue); 1: Elution fraction 100 mM imidazole; 2: Elution fraction 300 mM imidazole; 3: Elution fraction 500 mM imidazole. (B) Coomassie gel of CP-fusion proteins; M: Molecular marker (See blue); 1: RhoA-d25CP construct; 2: RhoA-FL-d25CP construct. (TIFF 909 kb)
Rights and permissions
About this article
Cite this article
Ortega-Berlanga, B., Musiychuk, K., Shoji, Y. et al. Engineering and expression of a RhoA peptide against respiratory syncytial virus infection in plants. Planta 243, 451–458 (2016). https://doi.org/10.1007/s00425-015-2416-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-015-2416-z