Abstract
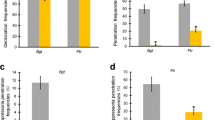
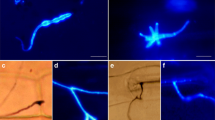
Non-host resistance (NHR) confers plant species immunity against the majority of microbes. As an important crop, wheat can be damaged by several Puccinia species but is immune to all Uromyces species. Here, we studied the basis of NHR in wheat against the broad bean rust pathogen Uromyces fabae (Uf). In the wheat–Uf interaction, microscopic observations showed that urediospores germinated efficiently on wheat leaves. However, over 98% of the germ tubes failed to form appressoria over stomata. For the few that invaded through stomata, the majority of them failed to penetrate wheat mesophyll cells. At 96 hours after inoculation, less than 4% of the Uf infection units that had entered the mesophyll tissue formed haustoria. Attempted penetration by haustorium mother cells induced the thickening of cell wall and the formation of papillae in plant cells, which arrested the development or growth of Uf penetration pegs. For the Uf haustoria formed in wheat cells, they were encased in callose-like materials and did not elicit hypersensitive response. Localized accumulation of H2O2 were observed in plant cell walls, papillae and encasement of haustoria during the wheat–Uf interaction. Furthermore, quantitative RT-PCR analysis showed that several genes involved in basal resistance and oxidative stress responses were up-regulated during Uf infection. In conclusion, our study revealed the cytological and molecular bases of NHR in wheat against the non-adapted rust fungus Uf, and highlighted the significance of papilla production in the prehaustorial NHR.








Similar content being viewed by others
Abbreviations
- CWA:
-
Cell wall apposition
- DAB :
-
3,3-diaminobenzidine
- DIC:
-
Differential interference contrast
- dai:
-
Days after inoculation
- hai:
-
Hours after inoculation
- HMC:
-
Haustorial mother cell
- HR:
-
Hypersensitive response
- MAMPS or PAMPs:
-
Microbial- or pathogen-associated molecular patterns
- MVBs:
-
Multivesicular bodies
- NBT:
-
Nitroblue tetrazolium
- NHR:
-
Non-host resistance
- Pgt :
-
P. graminis f. sp. tritici
- PRRs:
-
Pattern recognition receptors
- Pst :
-
P. striiformis f. sp. tritici
- Pt :
-
P. triticina
- PTI:
-
PAMP-triggered immunity
- qRT-PCR:
-
Quantitative RT-PCR
- ROS:
-
Reactive oxygen species
- SA:
-
Salicylic acid
- TaAPX :
-
Ascorbate-peroxidase
- TaCAT :
-
Catalase
- TaPAL :
-
Phenylalanine ammonia lyase
- TaPR1 :
-
Pathogenesis-related protein 1
- TaPR2 :
-
β-1,3-glucanase
- TaPR3 :
-
Chitinase
- TaPR5 :
-
Thaumatin-like
- TaSOD :
-
Superoxide dismutase
- TEM:
-
Transmission electron microscope
- Uf :
-
Uromyces fabae
References
Adhikari TB, Bai JF, Meinhardt SW, Gurung S, Myrfield M, Patel J et al (2009) Tsn1-mediated host responses to ToxA from Pyrenophora tritici-repentis. Mol Plant Microbe Interact 22:1056–1068
Allen EA, Hazen BE, Hoch HC, Kwon Y, Leinhos GME, Staples RC et al (1991) Appressorium formation in response to topographical signals by 27 rust species. Phytopathology 81:323–331
Bhuiyan N, Selvaraj G, Wei YD, King J (2009) Gene expression profiling and silencing reveal that monolignol biosynthesis plays a critical role in penetration defence in wheat against powdery mildew invasion. J Exp Bot 60:509–521
Collins NC, Thordal-Christensen H, Lipka V, Bau S, Kombrink E, Qiu JL et al (2003) SNARE-protein-mediated disease resistance at the plant cell wall. Nature 425:973–977
Coram TE, Wang MN, Chen XM (2008) Transcriptome analysis of the wheat–Puccinia striiformis f. sp tritici interaction. Mol Plant Pathol 9:157–169
Croxdale JL (2000) Stomatal patterning in angiosperms. Am J Bot 87:1069–1080
Deising HB, Werner S, Wernitz M (2000) The role of fungal appressoriain plant infection. Microbes Infect 2:1631–1641
Freialdenhoven A, Peterhansel C, Kurth J, Kreuzaler F, Schulze-Lefert P (1996) Identification of genes required for the function of non-race-specific mlo resistance to powdery mildew in barley. Plant Cell 8:5–14
Hardham AR (2007) Cell biology of fungal and Oomycete infection of plants. In: Howard RJ, Gow NAR (eds) The Mycota VIII. Biology of the fungal cell, 2nd edn. Springer, Berlin, pp 251–290
Heath MC (1974) Light and electron microscope studies of the interactions of host and non-host plants with cowpea rust—Uromyces phaseoli var. vignae. Physiol Plant Pathol 4:403–408
Heath MC (1979) Partial characterization of the electron-opaque deposits formed in the non-host plant, French bean after cowpea rust infection. Physiol Plant Pathol 15:141–144
Heath MC (1981) Resistance of plants to rust infection. Phytopathology 71:971–974
Heath MC (2000) Nonhost resistance and nonspecific plant defences. Curr Opin Plant Biol 3:315–319
Hoogkamp TJH, Chen WQ, Niks RE (1998) Specificity of prehaustorial resistance to Puccinia hordei and to two inappropriate rust fungi in barley. Phytopathology 88:856–861
Huckelhoven R, Dechert C, Kogel KH (2001) Non-host resistance of barley is associated with a hydrogen peroxide burst at sites of attempted penetration by wheat powdery mildew fungus. Mol Plant Pathol 2:199–205
Hulber SH, Bai J, Fellers JP, Pacheco MG, Bowden RL (2007) Gene expression patterns in near isogenic lines for wheat rust resistance gene Lr34/Yr18. Phytopathology 97:1083–1093
Jafary H, Albertazzi G, Marcel TC, Niks RE (2008) High diversity of genes for nonhost resistance of barley to heterologous rust fungi. Genetics 178:2327–2339
Jones JDG, Dangl JL (2006) The plant immune system. Nature 444:323–329
Kang Z, Huang L, Buchenauer H (2002) Ultrastructural changes and localization of lignin and callose in compatible and incompatible interactions between wheat and Puccinia striiformis. J Plant Dis Prot 109:25–37
Kang ZS, Wan GY, Huang LL, Wei GR, Zhao J (2003) Histology and ultrastructure of incompatible combination between Puccinia striiformis and wheat cultivars with low reaction type resistance. Agric Sci China 2:1102–1113
Kolmer JA, Ordonez ME, Groth JV (2009) The rust fungi. In: Encyclopedia of life sciences (ELS). Wiley, Chichester. doi:10.1002/9780470015902.a0021264
Laisk A, Oja V, Kull K (1980) Statistical distribution of stomatal apertures of Vicia faba and Hordeum vulgare and the Spannungsphase of stomatal opening. J Exp Bot 31:49–58
Lin KC, Bushnell WR, Szabo LJ, Smith AG (1996) Isolation and expression of a host response gene family encoding thaumatin-like proteins in incompatible oat-stem rust fungus interactions. Mol Plant Microbe Interact 9:511–522
Lipka V, Dittgen J, Bednarek P, Bhat R, Wiermer M, Stein M et al (2005) Pre- and postinvasion defences both contribute to nonhost resistance in Arabidopsis. Science 310:1180–1183
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25:402–408
Loehrer M, Langenbach C, Goellner K, Conrath U, Schaffrath U (2008) Characterization of nonhost resistance of Arabidopsis to the Asian soybean rust. Mol Plant Microbe Interact 21:1421–1430
Mauch F, Mauch-Mani B, Boller T (1988) Antifungal hydrolases in pea tissue. II. Inhibition of fungal growth by combinations of chitinase and β-1, 3-glucanase. Plant Physiol 88:936–942
Mellersh DG, Heath MC (2003) An investigation into the involvement of defense signaling pathways in components of the nonhost resistance of Arabidopsis thaliana to rust fungi also reveals a model system for studying rust fungal compatibility. Mol Plant Microbe Interact 16:398–404
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Mysore KS, Ryu CM (2004) Nonhost resistance: how much do we know? Trends Plant Sci 9:97–104
Niks RE (1983a) Comparative histology of partial resistance and the nonhost reaction to leaf rust pathogens in barley and wheat seedlings. Phytopathology 73:60–64
Niks RE (1983b) Haustorium formation by Puccinia hordei in leaves of hypersensitive, partially resistant, and nonhost plant genotypes. Phytopathology 73:64–66
Niks RE, Marcel TC (2009) Non-host and basal resistance: how to explain specificity? New Phytol 182:817–828
Orczyk W, Dmochowska-Boguta M, Czembor HJ, Nadolska-Orczyk A (2010) Spatiotemporal patterns of oxidative burst and micronecrosis in resistance of wheat to brown rust infection. Plant Pathol 59:567–575
Pritsch C, Muehlbauer GJ, Bushnell WR, Somers DA, Vance CP (2000) Fungal development and induction of defense response genes during early infection of wheat spikes by Fusarium graminearum. Mol Plant Microbe Interact 13:159–169
Rodrigues P, Garrood JM, Shen QH, Smith PH, Boyd LA (2004) The genetics of non-host disease resistance in wheat to barley yellow rust. Theor Appl Genet 109:425–432
Schaffrath U, Freydl E, Dudler R (1997) Evidence for different signaling pathways activated by inducers of acquired resistance in wheat. Mol Plant Microbe Interact 10:779–783
Schweizer P (2007) Nonhost resistance of plants to powdery mildew—new opportunities to unravel the mystery. Physiol Mol Plant Pathol 70:3–7
Shafiei R, Hang C, Kang JG, Loake GJ (2007) Identification of loci controlling non-host disease resistance in Arabidopsis against the leaf rust pathogen Puccinia triticina. Mol Plant Pathol 8:773–784
Sillero JC, Rubiales D (2002) Histological characterization of resistance to Uromyces viciae-fabae in faba bean. Phytopathology 92:294–299
Southerton SG, Deverall BJ (1990) Histochemical and chemical evidence for lignin accumulation during the expression of resistance to leaf rust fungi in wheat. Physiol Mol Plant Pathol 36:483–494
Stein M, Dittgen J, Sanchez-Rodriguez C, Hou BH, Molina A, Schulze-Lefert P et al (2006) Arabidopsis PEN3/PDR8, an ATP cassette transporter, contributes to nonhost resistance to inappropriate pathogens that enter by direct penetration. Plant Cell 18:731–746
Thordal-Christensen H (2003) Fresh insight into processes of nonhost resistance. Curr Opin Plant Biol 6:351–357
Thordal-Christensen H, Zhang Z, Wei YD, Collinge DB (1997) Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley–powdery mildew interaction. Plant J 11:1187–1194
Tufan HA, McGrann GRD, Magusin A, Morel JB, Miché L, Boyd LA (2009) Wheat Blast: histopathology and transcriptome reprogramming in response to adapted and non-adapted Magnaporthe isolates. New Phytol 184:473–484
Van Loon LC, Van Strien EA (1999) The families of pathogenesis-related proteins, their activities, and comparative analysis of PR-1 type proteins. Physiol Mol Plant Pathol 55:85–97
Voegele RT (2006) Uromyces fabae: development, metabolism, and interactions with its host Vicia faba. FEMS Microbiol Lett 259:165–173
Wang CF, Huang LL, Buchenauer H, Han QM, Zhang HC, Kang ZS (2007) Histochemical studies of the accumulation of reactive oxygen species(O2 − and H2O2) in the incompatible and compatible interaction of wheat–Puccinia striiformis f.sp. tritici. Physiol Mol Plant Pathol 71:230–239
Wang XJ, Tang CL, Zhang G, Li YC, Wang CF, Liu B et al (2009) cDNA-AFLP analysis reveals differential gene expression in compatible interaction of wheat challenged with Puccinia striiformis f. sp. tritici. BMC Genomics 10:289
Whetten R, Sederoff R (1995) Lignin biosynthesis. Plant Cell 7:1001–1013
Yu XM, Wang XJ, Wang CF, Chen XM, Qu ZP, Yu XD et al (2010) Wheat defense genes in fungal (Puccinia striiformis) infection. Funct Integr Genomics 10:227–239
Zipfel C (2008) Pattern-recognition receptors in plant innate immunity. Curr Opin Immunol 20:10–16
Acknowledgments
This study was supported by grants from National Basic Research Program of China (No. 2006CB100203), Modern Agro-industry Technology Research System in China, the National Natural Science Foundation of China (No. 30930064), and the 111 Project from the Ministry of Education of China (B07049).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, H., Wang, C., Cheng, Y. et al. Histological and molecular studies of the non-host interaction between wheat and Uromyces fabae . Planta 234, 979–991 (2011). https://doi.org/10.1007/s00425-011-1453-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-011-1453-5