Abstract
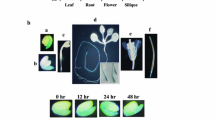
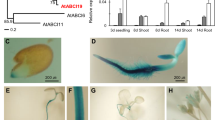
The phytohormone abscisic acid (ABA) regulates essential growth and developmental processes in plants. Recently, RNA-binding proteins have been described as components of ABA signaling during germination. We have identified ten ABA-regulated RNA-binding proteins in Arabidopsis seedlings. Among those genes, AtCSP41B and cpRNP29 are highly expressed in seedlings. Using promoter:reporter gene analyses, we showed that both AtCSP41B and cpRNP29 were in particular expressed in photosynthetically active organs like green cotyledons, leaves, and petioles. The analysis of CFP-fusion proteins demonstrates that cpRNP29 localized to chloroplasts and AtCSP41B to chloroplasts and stromules. Whereas RNA-binding of cpRNP29 has previously been shown, we demonstrated through in vitro RNA-binding assays that recombinant AtCSP41B binds to RNA, and that chloroplast petD RNA can serve as a target of AtCSP41B. Developmental or environmental stimuli affected the expression of AtCSP41B and cpRNP29 in seedlings. Both genes were repressed during senescence, but only AtCSP41B was significantly repressed upon water stress. In addition, AtCSP41B and cpRNP29 exhibited low expression in etiolated seedlings compared to green seedlings, and cpRNP29 was regulated during the day photoperiod. Homozygous T-DNA insertion lines were isolated, characterized on the molecular level, and monitored for phenotypic changes. Taken together, the data show that both proteins are regulated during processes that are known to involve ABA signaling. Their localization in chloroplasts and RNA-binding activity suggest a role in chloroplast RNA metabolism in Arabidopsis seedlings.







Similar content being viewed by others
Abbreviations
- ABA:
-
Abscisic acid
- AtCSP41B:
-
Arabidopsis thaliana chloroplast stem-loop-binding protein of 41 kDa
- CFP:
-
Cyan fluorescent protein
- cpRNP29:
-
Chloroplast ribonucleoprotein 29
- GFP:
-
Green fluorescent protein
- GUS:
-
Beta-glucuronidase
- NW:
-
Northwestern
- PPR:
-
Pentatricopeptide repeat
References
Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, Gadrinab C, Heller C, Jeske A, Koesema E, Meyers CC, Parker H, Prednis L, Ansari Y, Choy N, Deen H, Geralt M, Hazari N, Hom E, Karnes M, Mulholland C, Ndubaku R, Schmidt I, Guzman P, Aguilar-Henonin L, Schmid M, Weigel D, Carter DE, Marchand T, Risseeuw E, Brogden D, Zeko A, Crosby WL, Berry CC, Ecker JR (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301:653–657
Baginsky S, Gruissem W (2002) Endonucleolytic activation directs dark-induced chloroplast mRNA degradation. Nucleic Acids Res 30:4527–4533
Birkenmeier GF, Ryan CA (1998) Wound signaling in tomato plants. Evidence that ABA is not a primary signal for defense gene activation. Plant Physiol 117:687–693
Bollenbach TJ, Stern DB (2003) Secondary structures common to chloroplast mRNA 3′-untranslated regions direct cleavage by CSP41, an endoribonuclease belonging to the short chain dehydrogenase/reductase superfamily. J Biol Chem 278:25832–25838
Bollenbach TJ, Tatman DA, Stern DB (2003) CSP41a, a multifunctional RNA-binding protein, initiates mRNA turnover in tobacco chloroplasts. Plant J 36:842–852
Christmann A, Hoffmann T, Teplova I, Grill E, Muller A (2005) Generation of active pools of abscisic acid revealed by in vivo imaging of water-stressed Arabidopsis. Plant Physiol 137:209–219
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743
Duque P, Chua NH (2003) IMB1, a bromodomain protein induced during seed imbibition, regulates ABA- and phyA-mediated responses of germination in Arabidopsis. Plant J 35:787–799
Escobar NM, Haupt S, Thow G, Boevink P, Chapman S, Oparka K (2003) High-throughput viral expression of cDNA-green fluorescent protein fusions reveals novel subcellular addresses and identifies unique proteins that interact with plasmodesmata. Plant Cell 15:1507–1523
Fedoroff NV (2002) RNA-binding proteins in plants: the tip of an iceberg? Curr Opin Plant Biol 5:452–459
Freire MA, Pages M (1995) Functional characteristics of the maize RNA-binding protein MA16. Plant Mol Biol 29:797–807
Gomez J, Sanchez-Martinez D, Stiefel V, Rigau J, Puigdomenech P, Pages M (1988) A gene induced by the plant hormone abscisic acid in response to water stress encodes a glycine-rich protein. Nature 334:262–264
Hanahan D (1983) Studies on transformation of Escherichia coli with plasmids. J Mol Biol 166:557–580
Harmer SL, Hogenesch JB, Straume M, Chang HS, Han B, Zhu T, Wang X, Kreps JA, Kay SA (2000) Orchestrated transcription of key pathways in Arabidopsis by the circadian clock. Science 290:2110–2113
Heintzen C, Nater M, Apel K, Staiger D (1997) AtGRP7, a nuclear RNA-binding protein as a component of a circadian-regulated negative feedback loop in Arabidopsis thaliana. Proc Natl Acad Sci USA 94:8515–8520
Himmelbach A, Yang Y, Grill E (2003) Relay and control of abscisic acid signaling. Curr Opin Plant Biol 6:470–479
Holsters M, Silva B, Van Vliet F, Genetello C, De Block M, Dhaese P, Depicker A, Inze D, Engler G, Villarroel R et al (1980) The functional organization of the nopaline A. tumefaciens plasmid pTiC58. Plasmid 3:212–230
Hoth S, Morgante M, Sanchez JP, Hanafey MK, Tingey SV, Chua NH (2002) Genome-wide gene expression profiling in Arabidopsis thaliana reveals new targets of abscisic acid and largely impaired gene regulation in the abi1–1 mutant. J Cell Sci 115:4891–4900
Kim YO, Kim JS, Kang H (2005) Cold-inducible zinc finger-containing glycine-rich RNA-binding protein contributes to the enhancement of freezing tolerance in Arabidopsis thaliana. Plant J 42:890–900
Kohler RH, Hanson MR (2000) Plastid tubules of higher plants are tissue-specific and developmentally regulated. J Cell Sci 113(Pt 1):81–89
Kost B, Spielhofer P, Chua NH (1998) A GFP-mouse talin fusion protein labels plant actin filaments in vivo and visualizes the actin cytoskeleton in growing pollen tubes. Plant J 16:393–401
Kotera E, Tasaka M, Shikanai T (2005) A pentatricopeptide repeat protein is essential for RNA editing in chloroplasts. Nature 433:326–330
Kuhn JM, Schroeder JI (2003) Impacts of altered RNA metabolism on abscisic acid signaling. Curr Opin Plant Biol 6:463–469
Kusnetsov V, Herrmann RG, Kulaeva ON, Oelmuller R (1998) Cytokinin stimulates and abscisic acid inhibits greening of etiolated Lupinus luteus cotyledons by affecting the expression of the light-sensitive protochlorophyllide oxidoreductase. Mol Gen Genet 259:21–28
Kwok EY, Hanson MR (2004) Plastids and stromules interact with the nucleus and cell membrane in vascular plants. Plant Cell Rep 23:188–195
Li G, Bishop KJ, Chandrasekharan MB, Hall TC (1999) Beta-phaseolin gene activation is a two-step process: PvALF- facilitated chromatin modification followed by abscisic acid-mediated gene activation. Proc Natl Acad Sci USA 96:7104–7109
Lim MH, Kim J, Kim YS, Chung KS, Seo YH, Lee I, Kim J, Hong CB, Kim HJ, Park CM (2004) A new Arabidopsis gene, FLK, encodes an RNA binding protein with K homology motifs and regulates flowering time via FLOWERING LOCUS C. Plant Cell 16:731–740
Lorkovic ZJ, Barta A (2002) Genome analysis: RNA recognition motif (RRM) and K homology (KH) domain RNA-binding proteins from the flowering plant Arabidopsis thaliana. Nucleic Acids Res 30:623–635
Lurin C, Andres C, Aubourg S, Bellaoui M, Bitton F, Bruyere C, Caboche M, Debast C, Gualberto J, Hoffmann B, Lecharny A, Le Ret M, Martin-Magniette ML, Mireau H, Peeters N, Renou JP, Szurek B, Taconnat L, Small I (2004) Genome-wide analysis of Arabidopsis pentatricopeptide repeat proteins reveals their essential role in organelle biogenesis. Plant Cell 16:2089–2103
Macknight R, Bancroft I, Page T, Lister C, Schmidt R, Love K, Westphal L, Murphy G, Sherson S, Cobbett C, Dean C (1997) FCA, a gene controlling flowering time in Arabidopsis, encodes a protein containing RNA-binding domains. Cell 89:737–745
Meierhoff K, Felder S, Nakamura T, Bechtold N, Schuster G (2003) HCF152, an Arabidopsis RNA binding pentatricopeptide repeat protein involved in the processing of chloroplast psbB-psbT-psbH-petB-petD RNAs. Plant Cell 15:1480–1495
Mockler TC, Yu X, Shalitin D, Parikh D, Michael TP, Liou J, Huang J, Smith Z, Alonso JM, Ecker JR, Chory J, Lin C (2004) Regulation of flowering time in Arabidopsis by K homology domain proteins. Proc Natl Acad Sci USA 101:12759–12764
Nakamura T, Meierhoff K, Westhoff P, Schuster G (2003) RNA-binding properties of HCF152, an Arabidopsis PPR protein involved in the processing of chloroplast RNA. Eur J Biochem 270:4070–4081
Nakamura T, Ohta M, Sugiura M, Sugita M (2001) Chloroplast ribonucleoproteins function as a stabilizing factor of ribosome-free mRNAs in the stroma. J Biol Chem 276:147–152
Nakamura T, Schuster G, Sugiura M, Sugita M (2004) Chloroplast RNA-binding and pentatricopeptide repeat proteins. Biochem Soc Trans 32:571–574
Ohta M, Sugita M, Sugiura M (1995) Three types of nuclear genes encoding chloroplast RNA-binding proteins (cp29, cp31 and cp33) are present in Arabidopsis thaliana: presence of cp31 in chloroplasts and its homologue in nuclei/cytoplasms. Plant Mol Biol 27:529–539
Papp I, Mur LA, Dalmadi A, Dulai S, Koncz C (2004) A mutation in the Cap Binding Protein 20 gene confers drought tolerance to Arabidopsis. Plant Mol Biol 55:679–686
Rousseaux MC, Hall AJ, Sanchez RA (1996) Far-red enrichment and photosynthetically active radiation level influence leaf senescence in field-grown sunflower. Physiol Plant 96:217–224
Sato S, Nakamura Y, Kaneko T, Asamizu E, Tabata S (1999) Complete structure of the chloroplast genome of Arabidopsis thaliana. DNA Res 6:283–290
Seki M, Narusaka M, Kamiya A, Ishida J, Satou M, Sakurai T, Nakajima M, Enju A, Akiyama K, Oono Y, Muramatsu M, Hayashizaki Y, Kawai J, Carninci P, Itoh M, Ishii Y, Arakawa T, Shibata K, Shinagawa A, Shinozaki K (2002) Functional annotation of a full-length Arabidopsis cDNA collection. Science 296:141–145
Simpson GG, Quesada V, Henderson IR, Dijkwel PP, Macknight R, Dean C (2004) RNA processing and Arabidopsis flowering time control. Biochem Soc Trans 32:565–566
Stadler R, Wright KM, Lauterbach C, Amon G, Gahrtz M, Feuerstein A, Oparka KJ, Sauer N (2005) Expression of GFP-fusions in Arabidopsis companion cells reveals non-specific protein trafficking into sieve elements and identifies a novel post-phloem domain in roots. Plant J 41:319–331
Staiger D, Zecca L, Wieczorek Kirk DA, Apel K, Eckstein L (2003) The circadian clock regulated RNA-binding protein AtGRP7 autoregulates its expression by influencing alternative splicing of its own pre-mRNA. Plant J 33:361–371
Stern DB, Gruissem W (1987) Control of plastid gene expression: 3′ inverted repeats act as mRNA processing and stabilizing elements, but do not terminate transcription. Cell 51:1145–1157
Susek RE, Ausubel FM, Chory J (1993) Signal transduction mutants of arabidopsis uncouple nuclear CAB and RBCS gene expression from chloroplast development. Cell 74:787–799
Takahashi S, Seki M, Ishida J, Satou M, Sakurai T, Narusaka M, Kamiya A, Nakajima M, Enju A, Akiyama K, Yamaguchi-Shinozaki K, Shinozaki K (2004) Monitoring the expression profiles of genes induced by hyperosmotic, high salinity, and oxidative stress and abscisic acid treatment in Arabidopsis cell culture using a full-length cDNA microarray. Plant Mol Biol 56:29–55
Toth Z, Lischka P, Stamminger T (2006) RNA-binding of the human cytomegalovirus transactivator protein UL69, mediated by arginine-rich motifs, is not required for nuclear export of unspliced RNA. Nucleic Acids Res 34:1237–1249
van Wijk KJ (2004) Plastid proteomics. Plant Physiol Biochem 42:963–977
Yamaguchi-Shinozaki K, Shinozaki K (2005) Organization of cis-acting regulatory elements in osmotic- and cold-stress-responsive promoters. Trends Plant Sci 10:88–94
Yang J, Schuster G, Stern DB (1996) CSP41, a sequence-specific chloroplast mRNA binding protein, is an endoribonuclease. Plant Cell 8:1409–1420
Yang J, Stern DB (1997) The spinach chloroplast endoribonuclease CSP41 cleaves the 3′-untranslated region of petD mRNA primarily within its terminal stem-loop structure. J Biol Chem 272:12874–12880
Yang J, Zhang J, Wang Z, Zhu Q, Liu L (2002) Abscisic acid and cytokinins in the root exudates and leaves and their relationship to senescence and remobilization of carbon reserves in rice subjected to water stress during grain filling. Planta 215:645–652
Yohn CB, Cohen A, Danon A, Mayfield SP (1998) A poly(A) binding protein functions in the chloroplast as a message-specific translation factor. Proc Natl Acad Sci USA 95:2238–2243
Zapata JM, Guera A, Esteban-Carrasco A, Martin M, Sabater B (2005) Chloroplasts regulate leaf senescence: delayed senescence in transgenic ndhF-defective tobacco. Cell Death Differ 12(10):1277–1284
Zimmermann P, Hirsch-Hoffmann M, Hennig L, Gruissem W (2004) GENEVESTIGATOR. Arabidopsis microarray database and analysis toolbox. Plant Physiol 136:2621–2632
Zuker M (2003) Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res 31:3406–3415
Acknowledgments
We thank Petra Dietrich, Ulrich Hammes, and Norbert Sauer for comments on the manuscript, Franz Klebl for support with real-time RT-PCRs, and Susanne Schrack and Stefanie Geigner for excellent technical assistance. We thank Peter Hare and Nam-Hai Chua (Rockefeller University, New York) for providing the CFP-pBA002 vector, the Riken Biosource Center for providing the RAFL cDNA clones, and the SALK Institute for providing seeds for the T-DNA insertion lines. This work was supported by the Deutsche Forschungsgemeinschaft (through grants to S.H. and T.S. in the SFB473).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Raab, S., Toth, Z., de Groot, C. et al. ABA-responsive RNA-binding proteins are involved in chloroplast and stromule function in Arabidopsis seedlings. Planta 224, 900–914 (2006). https://doi.org/10.1007/s00425-006-0282-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-006-0282-4