Abstract
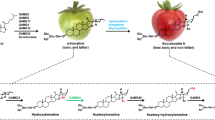
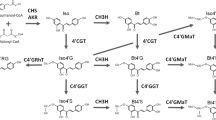
Saffron, the dry stigma of Crocus sativus L., is considered to be the world’s most expensive spice. Three major apocarotenoids—crocin, crocetin and picrocrocin—are responsible for the colour and bitter taste of saffron. The final step in the biosynthesis of the 20-carbon esterified carotenoid crocin is the transformation of the insoluble crocetin into a soluble and stable storage form by glucosylation. These glucosylation reactions are catalysed by glucosyltransferases (GTases) that play a crucial role in natural-product biosynthesis. Using degenerate primers designed to match the plant secondary product GTase (PSPG) box we cloned two cDNAs, UGTCs2 and UGTCs3, from C. sativus stigmas that encode putative polypeptides of 460 and 475 amino acids, respectively. These genes were expressed differentially in saffron tissues. UGTCs2 was mainly expressed in fully developed stigmas, whereas UGTCs3 was mainly expressed in stamens. The UGTCs2 transcript was not detected in the stigma tissue of a Crocus species that does not synthesize crocin, while UGTCs3 and other structural genes for carotenoid biosynthesis were expressed in the stigma of all tested Crocus species. To identify the biochemical function of UGTCs2, the isolated cDNA was expressed in Escherichia coli cells. The recombinant protein UGTCs2 had glucosylation activity against crocetin, crocetin β-d-glucosyl ester and crocetin β-d-gentibiosyl ester. These results might suggest that the isolated clone UGTCs2 codes for a saffron crocetin GTase.






Similar content being viewed by others
Abbreviations
- ABA :
-
Abscisic acid
- BCH :
-
β-Carotene hydroxylase
- IAA :
-
Indole acetic acid
- GA 3 :
-
Gibberellic acid
- GTase :
-
Glucosyltransferase
- JA :
-
Jasmonic acid
- PSY :
-
Phytoene synthase
- RACE :
-
Rapid amplification of cDNA ends
- SA :
-
Salicylic acid
- UGT :
-
UDP-glucosyl transferase
References
Abdullaev FI (2002) Cancer chemopreventive and tumoricidal properties of saffron (Crocus sativus). Exp Biol Med 227:20–25
Alonso GL, Salinas MR, Garijo J (1998) Method to determine the authenticity of aroma of saffron (Crocus sativus L). J Food Prot 61:1525–1528
Bowles DJ (1998) A novel tomato gene that rapidly responds to wound- and pathogen-related signals. Plant J 14:137–142
Boss PK, Davies C, Robinson SP (1996) Expression of anthocyanin biosynthesis pathway genes in red and white grapes. Plant Mol Biol 32:565–569
Bouvier F, Suire C, Mutterer J, Camara B (2003) Oxidative remodeling of chromoplast carotenoids: identification of the carotenoid dioxygenase CsCCD and CsZCD genes involved in Crocus secondary metabolite biogenesis. Plant Cell 15:47–62
Church GM, Gilbert W (1984) Genomic sequencing. Proc Natl Acad Sci USA 81:1991–1995
Cormier F, Dufresne C, Dorion S (1995) Enhanced crocetin glucosylation by means of maltosyl-β-cyclodextrin encapsulation. Biotechnol Tech 9:553–556
Côté F, Cormier F, Dufresne C, Willemot C (2000) Properties of a glucosyltransferase involved in crocin synthesis. Plant Sci 153:55–63
Dixon SC, Martin RC, Mok MC, Shaw G, Mok DWS (1989) Zeatin glycosylation enzymes in Phaseolus. Isolation of O-glucosyltransferase from P. lunatus and comparison to O-xylosyltransferase from P. vulgaris. Plant Physiol 90:1316–1321
Doyle JJ, Doyle LH (1990) Isolation of plant DNA from fresh tissue. Focus 12:13–15
Dufresne C, Cormier F, Dorion S (1997) In vitro formation of crocetin glucosyl esters by Crocus sativus callus extract. Planta Med 63:150–153
Dufresne C, Cormier F, Dorion S, Niggli UA, Pfister S, Pfander H (1999) Glycosylation of encapsulated crocetin by a Crocus sativus L. cell culture. Enzyme Microbial Technol 24:453–462
Eugster CH, Hurlimann H, Leuenberger HJ (1969) Crocetindyaldehyd und crocetinhaldaldehyb als blutenfarbstoffe von Jacquinia angustifolia. Helv Chim Acta 52:89–90
Ford CM, Boss P, Hoj PB (1998) Cloning and characterization of Vitis vitifera UDP-glucose:flavonoid 3-O-glucosyltransferase, a homologue of enzyme encoded by the maize Bronze-1 locus that may primarily serve to glucosylate anthocyanidins in vivo. J Biol Chem 273:9224–9233
Gopalakrishna R, Kumar G, Prasad BT, Mathew MK, Kumar M (2001) A stress-responsive gene from groundnut, Gdi-15, is homologous to flavonol 3-O-glucosyltransferase involved in anthocyanin biosynthesis. Biochem Biophys Res Commun 284:574–579
Himeno H, Sano K (1987) Synthesis of crocin, picrocrocin and safranal by saffron stigma-like structures proliferated in vitro. Agric Biol Chem 51:2395–2400
Horvath D, Chua NH (1996) Identification of an immediate-early salicylic acid-inducible tobacco gene and characterization of induction by other compounds. Plant Mol Biol 31:1061–1072
Hosseinzadeh H, Younesi HM (2002) Antinociceptive and anti-inflammatory effects of Crocus sativus L. stigma and petal extracts in mice. BMC Pharmacol 2:7–14
Hughes J, Hughes MA (1994) Multiple secondary plant product UDP-glucose glucosyltransferase genes expressed in cassava (Manihot esculenta Crantz) cotyledons. DNA Seq 5:41–49
Imanishi S, Hashizume K, Kojima H, Ichihara A, Nakamura K (1998) An mRNA of tobacco cell, which is rapidly inducible by methyl jasmonate in the presence of cycloheximide, codes for a putative glycosyltransferase. Plant Cell Physiol 39:202–211
Jones PR, Møller BL, Høj PB (1999) The UDP-glucose:p-hydroxy-mandelonitrile-O-glucosyltransferase that catalyses the last step in synthesis of the cyanogenic glucoside dhurrin in Sorghum bicolor. J Biol Chem 274:35483–35491
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lee H-I, Raskin I (1999) Purification, cloning, and expression of a pathogen inducible UDP-glucose: salicylic acid glucosyltransferase from tobacco. J Biol Chem 274:36637–36642
Li Y, Baldauf S, Lim E-K, Bowles D (2001) Phylogenetic analysis of the UDP-glycosyltransferase multigene family of Arabidopsis thaliana. J Biol Chem 276:4338–4343
Liao YH, Houghton PJ, Hoult JR (1999) Novel and known constituents from Buddleja species and their activity against leukocyte eicosanoid generation. J Nat Prod 62:1241–1245
Lim EK, Li Y, Parr A, Jackson R, Ashford DA, Bowles DJ (2001) Identification of glucosyltransferase genes involved in sinapate metabolism and lignin synthesis in Arabidopsis. J Biol Chem 276:4344–4349
Lim E-K, Doucet CJ, Li Y, Elias L, Worrall D, Spencer SP, Ross J, Bowles DJ (2002) The activity of Arabidopsis glycosyltransferases toward salicylic acid, 4-hydroxybenzoic acid, and other benzoates. J Biol Biochem 277:586–592
Lim E-K, Baldauf S, Li Y, Elias L, Worrall D, Spencer SP, Jackson, RG, Taguchi G, Ross J, Bowles DJ (2003) Evolution of substrate recognition across a multigene family of glycosyltransferases in Arabidopsis. Glycobiology 13:139–145
Miller KD, Guyon V, Evans JN, Shuttleworth WA, Taylor LP (1999) Purification, cloning, and heterologous expression of a catalytically efficient flavonol 3-O-galactosyltransferase expressed in the male gametophyte of Petunia hybrida. J Biol Chem 274:34011–34019
Pfander H (1976) carotenoid glycosides. Pure Appl Chem 47:121–128
Pfander H, Wittwer F (1975) Carotinoid-glykoside. Unterguchungen zur carotinoids zusammensetzung im safran. Helv Chim Acta 58:1608–1620
Pfander H, Wittwer F (1979) Carotinoid-Glycosylester (3. Mitteilung). Die Synthese von Crocetin-di-(beta-d-glucosyl)-ester. Eine neue Methode zur selektiven Veresterung von ungeschützter beta-d-Glucose. Helv Chim Acta 62:1944–1951
Pfander H, Schurtenberger H (1982) Biosynthesis of C20-carotenoids in Crocus sativus. Phytochemistry 21:1039–1042
Pfister S, Meyer P, Steck A, Pfander H (1996) Isolation and structure elucidation of carotenoid glycoslyesters on gardenia fruits (Gardenia jasminoides) and saffron (Crocus sativus) J Agric Food Chem 44:2612–2615
Rea PA (1999) MRP subfamily ABC transporters from plant and yeast. J Exp Bot 50:895–913
Ross J, Li Y, Lim E, Bowles DJ (2001) Higher plant glycosyltransferases. Genome Biol 2:3004.1–3004.6
Rychener M, Bigler P, Pfander H (1984) Isolation and structure elucidation of neapolitanose (O-β-d-glucopyranosyl-(1→2)-O-[β-d-glucopyranosyl-(1→6)[-d-glucose), a new trisaccharide from the stigmas of garden crocuses (Crocus neapolitanus var.) Helv Chim Acta 67:386–556
Song C (1991) Chemical constituents of saffron (Crocus sativus) II. The flavonol compounds of petals. Chem Abstr 114:214–242
Straubinger M, Jezussek M, Waibel R, Winterhalter P (1997) Novel glycosidic constituents of saffron. J Agric Food Chem 45:1678–1681
Szerszen JB, Szczyglowski K, Bandurski RS (1994) Iaglu, a gene from Zea mays involved in conjugation of growth hormone indole-3-acetic acid. Science 265:1699–1701
Tandon JS, Katti SB, Ruedi P, Eugster CH (1979) Crocetin-dialdehyde from Coleus forskohlii Briq., labiatae. Helv Chim Acta 274:2706–2707
Tarantilis PA, Polissiou, M (1997) Isolation and identification of the aroma constituents of saffron (Crocus sativa). J Agric Food Chem 45:459–462
Tarantilis PA, Tsoupras G, Polissiou M (1995) Determination of saffron (Crocus sativus L.) components in crude plant extract using high-performance liquid chromatography-UV-visible photodiode-array detection-mass spectrometry. J Chromatogr 699:107–118
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4573–4680
Vogt T, Jones P (2000) Glycosyltransferases in plant natural product synthesis: characterization of a supergene family. Trends Plant Sci 5:380–386
Vogt T, Taylor LP (1995) Flavonol 3-O-glycosyltransferases associated with petunia pollen produce gametophyte-specific flavonol diglycosides. Plant Physiol 108:903–911
Verma SK, Bordia A (1998) Antioxidant property of saffron in man. Indian J Med Sci 52:205–207
Wierman R, Vieth K (1983) Outer pollen wall, an important accumulation site for flavonoids. Protoplasma 118:230–233
Winterhalter P, Rouseff RS, eds (2001) Carotenoid-derived aroma compounds: an introduction. In: Carotenoid-derived aroma compounds. American Chemical Society, Washington, DC, pp 1–17
Winterhalter P, Skouroumounis GK (1997) Glycoconjugated aroma compounds: occurrence, role and biotechnological transformation. Adv Biochem Eng Biotechnol 55:73–105
Yamazaki M, Gong Z, Fukichi-Mizutani, M, Fukui Y, Tanaka Y, Kusumi T, Saito K (1999) Molecular cloning and biochemical characterization of a novel anthocyanin 5-O-glucosyltransferase by mRNA differential display for plant forms regarding anthocyanin. J Biol Chem 274:7405–7411
Acknowledgements
We gratefully acknowledge the contribution of R. Castillo in the collection and preparation of some RNA samples. We thank Drs. A. Gómez and D. Lunt (Hull University, UK) for critical reading of the manuscript and corrections; the Biochemistry Department of Valencia University (Spain) for radiation laboratory facilities; and Drs. N. Kayali (Universidad Complutense de Madrid, Spain) and O. Jauregui (Barcelona University, Spain) for the mass spectra facilities and analysis. This research was supported by Consejería de Ciencia y Tecnología de la JCCM grant No. PAI-02-026 and MCyT grant BIO2003-05259.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moraga, A.R., Nohales, P.F., Pérez, J.A.F. et al. Glucosylation of the saffron apocarotenoid crocetin by a glucosyltransferase isolated from Crocus sativus stigmas. Planta 219, 955–966 (2004). https://doi.org/10.1007/s00425-004-1299-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-004-1299-1