Abstract
In contrast to oil seeds, potato (Solanum tuberosum L.) is characterized by a high amount of starch stored in the tubers. To assess the capacity for oil synthesis in potato tubers, the changes in lipid content and flux into lipid synthesis were explored in transgenic potatoes altered in carbohydrate or lipid metabolism. A strong decrease in the amount of starch observed in antisense lines for ADP-glucose pyrophosphorylase or plastidic phosphoglucomutase had no effect on storage-lipid content. Similarly, potato lines over-expressing the Arabidopsis thaliana (L.) Heynh. plastidic ATP/ADP transporter that contained an increased amount of starch were not altered in oil content, indicating that the plastidic ATP level is not limiting fatty acid synthesis in potato tubers. However, over-expression of the acetyl-CoA carboxylase from Arabidopsis in the amyloplasts of potato tubers led to an increase in fatty acid synthesis and a more than 5-fold increase in the amount of triacylglycerol. Taken together, these data demonstrate that potato tubers have the capacity for storage-lipid synthesis and that malonyl-CoA, the substrate for elongation during fatty acid synthesis, represents one of the limiting factors for oil accumulation.
Similar content being viewed by others
Introduction
In higher plants, carbohydrates synthesized during photosynthesis in leaves are transported to the storage organs, e.g. seeds or tubers. In these so-called “sink” organs, carbon is stored in the form of protein (e.g. soybean), oil (e.g. rapeseed, Arabidopsis) or starch (e.g. potato tubers, cereals). The distribution of carbon to the different forms of storage compounds can vary between plant species, and the mechanisms that control this process are to a large extent unknown. Plant tissues that usually do not accumulate high amounts of starch and oil still contain the enzyme machinery to synthesize these storage compounds. For example, developing seeds of Arabidopsis and rape accumulate intermediate amounts of starch, which later is converted to oil (Focks and Benning 1998). Plastids from cauliflower buds and from pea accumulate high amounts of starch, and in in vitro assays are capable of synthesizing high amounts of fatty acids as well (Denyer and Smith 1988; Möhlmann et al. 1994).
In potato tubers, the de novo synthesis of starch is localized to amyloplasts. Sucrose derived from the leaves is transported into the cytosol of tuber cells and converted to glucose-6-phosphate. After import into the amyloplast, glucose-6-phosphate is converted to ADP-glucose by subsequent action of the enzymes phosphoglucomutase and ADP-glucose-pyrophosphorylase. These two enzymes are crucial for the synthesis of starch in potato tubers, because the inhibition of either activity by antisense expression results in a drastic down-regulation of starch production (Müller-Röber et al. 1992; Tauberger et al. 2000). The activation of glucose by ADP-glucose-pyrophosphorylase is ATP-dependent. Therefore, high amounts of ATP must be transported from the cytosol into the stroma, a process mediated by the plastidic ATP/ADP transporter. By overexpression of the ATP/ADP transporter from Arabidopsis in potato it was shown that, indeed, this activity is limiting for starch synthesis (Tjaden et al. 1998).
In plants, de novo synthesis of fatty acids is also localized to the plastids. Pyruvate is considered the immediate precursor for fatty acid synthesis in most plastids (Kang and Rawsthorne 1994; Denyer and Smith 1988; Möhlmann et al. 1994; Schwender and Ohlrogge 2002). Pyruvate represents the end product of glycolysis and is converted to acetyl-CoA by pyruvate dehydrogenase. In the plastid, acetyl-CoA is the substrate for the acetyl-CoA carboxylase (ACCase) reaction, resulting in the synthesis of malonyl-CoA, the substrate for chain elongation during fatty acid de novo synthesis. ACCase, which exists in a cytosolic, multifunctional form as well as a plastidic, multi-subunit form, is presumed to be involved in the regulation of fatty acid synthesis in plants (Ohlrogge and Jaworski 1997). Overexpression of the cytosolic form of ACCase in plastids of transgenic rapeseed led to a small but significant increase in lipid content (Roesler et al. 1997). After export from the plastids, fatty acids are incorporated into glycerolipids at the endoplasmic reticulum via the Kennedy pathway. The synthesis of triacylglycerol, the most abundant form of storage lipid, is catalyzed at least in part by the acyl-CoA:diacylglycerol acyltransferase (DGAT1). An Arabidopsis mutant (AS11) carrying a mutation in the structural gene for DGAT1 is reduced in seed oil content (Katavic et al. 1995; Zou et al. 1999). Overexpression of the Arabidopsis enzyme in tobacco leaves and in Arabidopsis seeds resulted in an increased amount of triacylglycerol (Bouvier-Navé et al. 2000; Jako et al. 2001).
Because of its high per hectare yield, potato (Solanum tuberosum L.) represents one of the most productive crops in many regions of the world. Therefore, potato might be regarded as an ideal transgenic species for the production of a wide number of industrially important compounds, e.g. fatty acids and derivatives thereof. For this reason, it is desirable to explore in more detail the capacity of potato tubers to produce alternative products. In contrast to oil seeds such as rapeseed and sunflower, potato tubers contain high amounts of starch but are very low in oil. The total lipid content per unit fresh weight is about 0.1% (Galliard 1968). Whereas membrane lipids, in particular phospholipids and galactolipids, make up the predominant fraction of lipids in potato tubers, triacylglycerol was detected only in trace amounts (Pun et al. 1980). Furthermore, changes in lipid composition in the tubers were found during acclimation to low temperatures and during long periods of storage (Liljenberg et al. 1978; Palta et al. 1993; Spychalla 1994). To unravel the factors limiting oil synthesis in potato, we analyzed lipid content and fatty acid synthesis in several transgenic potato lines with alterations in starch and lipid synthesis. Because of their low endogenous lipid synthesis activity, potato tubers represent an ideal model to study the effects of alterations in enzyme activities on fatty acid accumulation. However, the changes in lipid content achieved in this study represent only the initial steps towards the attempt to re-direct a substantial amount of carbon from starch to lipid.
Materials and methods
Plant material
Wild type (Désirée) and transgenic potato plants (Solanum tuberosum L.) used for lipid analysis were grown in the greenhouse at 22°C and 16 h light per day. The following transgenic lines were employed: aAGP-93, antisense expression of ADP-glucose pyrophosphorylase (Müller-Röber et al. 1992); aPGMII-5, antisense expression of plastidic phosphoglucomutase (Tauberger et al. 2000); AATP-62, JT-62, overexpression of the plastidic ATP/ADP-transporter (Tjaden et al. 1998); DGAT1, overexpression of the Arabidopsis acyl-CoA:diacylglycerol acyltransferase (see below); ACC, overexpression of acetyl-CoA carboxylase from Arabidopsis (see below). Tubers for enzyme assays were collected 10 weeks after planting, and tubers used for lipid analysis were harvested at the end of the vegetative period.
Transgenic potato lines overexpressing ACCase (ACC1)
The isolation of the genomic clone for the homomeric acetyl-CoA carboxylase (ACC1) from Arabidopsis thaliana (L.) Heynh. was described elsewhere (Roesler et al. 1997). The ACC1 gene was ligated into the SmaI, SacI sites of pBI121 containing the cauliflower mosaic virus (CaMV) 35S promoter. For targeting into the chloroplasts of plants, this construct contained an N-terminal signal sequence extension from the small subunit of the soybean Rubisco cDNA (Jefferson et al. 1987; Roesler et al. 1997). Potato leaf discs were transformed with Agrobacterium harboring the respective binary vectors. Transgenic plants were regenerated according to Rocha-Sosa et al. (1989).
Northern and Western analysis
Total RNA from leaves of 6-week-old primary transformants was extracted according to Logemann et al. (1987). Northern blotting was carried out by separating RNA samples (10 μg) on formaldehyde gels and blotting onto nylon filters. Randomly primed 32P-labeled probes corresponding to the ACCase genomic clone were used for filter hybridization using standard protocols (Sambrook et al. 1989). Hybridization signals were detected by autoradiography. Proteins isolated from potato tubers were transferred to polyvinylidenedifluoride (PVDF) membranes and probed with anti-biotin antibodies as described by Roesler et al. (1997).
Lipid analysis
Total fatty acids were quantified in whole tissue by transmethylation with 1 N HCl in methanol. The fatty acid methyl esters were measured by gas chromatography according to Browse et al. (1986). Total lipids were separated into polar lipids, diacylglycerol, free fatty acids and triacylglycerol by thin-layer chromatography (TLC) on silica plates developed with hexane/diethyl ether/acetic acid (85:15:1, v/v). Lipids were isolated from the plates and quantified by GC after derivatization to fatty acid methyl esters according to Browse et al. (1986) using pentadecanoic acid (15:0) as internal standard. Polar lipids were analyzed as described in Dörmann et al. (1995).
Enzyme assays
Flux into fatty acid synthesis was measured by radiolabeling lipids in fresh potato tuber discs with 1 mM sodium [1-14C]acetate (74 MBq mmol−1) in 20 mM Mes–KOH, pH 6.0. After incubation, the tuber discs were washed, frozen in liquid nitrogen and ground in a porcelain mortar. After determining the weight, lipids were extracted with chloroform/methanol and the incorporation of acetate into the lipid phase quantified by scintillation counting.
ACCase activity was assayed as described by Roesler et al. (1996). Total protein extracts were prepared in 100 mM Tricine–KOH (pH 8.2), 100 mM KCl, 2.5 mM MgCl2, 1 mM EDTA, 1 mM DTT, 0.1% bovine serum albumin, filtered through two layers of Miracloth and centrifuged for 1 min at 10,000 g. Enzyme activity was measured with 100 μg protein in 100 µl of the described buffer, after addition of 1 mM acetyl-CoA, 1 mM ATP and 1 mM [14C]NaHCO3 (1.95 GBq mmol−1) for 30 min at 30°C. Incubations were stopped by adding 2 volumes of 2 N HCl and the samples taken to complete dryness by evaporation. Acid-stable products were dissolved in 50 μl H2O, and radioactivity determined by scintillation counting. Non-specific carboxylation in each extract was determined by subtracting the amount of radioactivity incorporated into acid-stable compounds in the absence of acetyl-CoA.
Activities of fatty acid synthesis were measured with [2-14C]malonyl-CoA according to McKeon and Stumpf (1982).
Results
Alterations in carbohydrate metabolism in transgenic potato tubers do not affect storage lipid content
Starch represents the major form of carbon stored in potato tubers. As a first approach to manipulate storage-product synthesis in potato tubers, three transgenic lines were examined with alterations in the flux of carbon into starch. To explore the hypothesis that an inhibition of starch synthesis can result in a re-direction of carbon to lipid synthesis, two antisense lines were analyzed which contained reduced activities of ADP-glucose pyrophosphorylase (aAGP-93; Müller-Röber et al. 1992) or of the plastidic form of phosphoglucomutase (aPGMII-5, Tauberger et al. 2000; Fig. 1). These two lines were reduced in ADP-glucose synthesis, and therefore were also reduced in starch. In addition, we examined a transgenic line in which over-expression of the plastidic ATP/ADP transporter resulted in an increased amount of plastidic ATP and an increased rate of ADP-glucose synthesis (line AATP-62; Tjaden et al. 1998; Geigenberger et al. 2001; Fig. 1). As a consequence, these lines contain a higher amount of starch. Because ATP is also required for the synthesis of malonyl-CoA, the substrate for plastidic fatty acid synthesis, an elevated amount of ATP might similarly stimulate lipid synthesis.
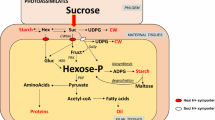
Generalized scheme of starch and lipid synthesis in potato (Solanum tuberosum) tubers. After phloem unloading, sucrose is converted to UDP-glucose and fructose in tuber cells by sucrose synthase. A large fraction of carbohydrate is imported into the amyloplast and used to produce ADP-glucose, the precursor for starch synthesis. A minor amount of carbohydrate is metabolized via glycolysis or converted to acetyl-CoA and malonyl-CoA for fatty acid de novo synthesis in the amyloplast. Fatty acyl groups are exported to the endoplasmic reticulum for lipid biosynthesis. Enzymatic steps and transporters analyzed in this study are boxed and highlighted. ACCase Acetyl-CoA carboxylase, AGP ADP-glucose pyrophosphorylase, ACP acyl carrier protein, AATP plastidic ATP/ADP-transporter, bP bisphosphate, CoA coenzyme A, DAG diacylglycerol, DGAT acyl-CoA:diacylglycerol acyltransferase, Frc fructose, Glc glucose, P phosphate, PGM plastidic phosphoglucomutase, TAG triacylglycerol
The lipid content of plant tissues can be determined by measuring total fatty acids by GC after derivatization to fatty acid methyl esters. No changes in the total fatty acid content per unit fresh weight or in the fatty acid composition were observed in the three transgenic lines with alterations in starch metabolism (Table 1). To determine the contents of individual lipid classes, total lipids were extracted from tubers, separated into different lipid classes by TLC and quantified by GC of fatty acid methyl esters. In wild-type potato tubers, membrane lipids constitute about 90 mol% of total lipids, whereas triacylglycerol amounts to only 1–1.1 mol% (Table 1). The lipid composition in the three transgenic lines was not changed.
The incorporation of radiolabeled [14C]acetate into the lipid fraction can be taken as a measure for the activity of de novo fatty acid synthesis in plants. To estimate the amount of radiolabel incorporated into the fatty acid fraction in wild-type tuber discs, the lipid phase was transmethylated and after separation by TLC, radioactivity was quantified by scintillation counting of isolated lipid bands. About 85% of total label co-migrated with fatty acid methyl esters, indicating that [14C]acetate was preferentially incorporated into fatty acids (data not shown). The radio-labeling of fatty acids with acetate was very similar for the wild type (WT) and the transgenic lines aAGP-93, aPGM-5 and AATP-62 (Fig. 2). Provided that the endogenous pools of acetate in the different transgenic lines are comparable, this represents corroborating evidence for the finding that carbon flux into fatty acids was not changed. Taken together, these results demonstrate that an inhibition in starch synthesis or an increase in the amount of plastidic ATP per se does not lead to an increased accumulation of total fatty acids or of the triacylglycerol fraction in potato tubers.
Lipid synthesis in tubers of transgenic potato plants affected in carbohydrate metabolism. Lipid synthesis in WT and transgenic lines was determined by incubating tuber discs with radioactive acetate. Lipids were extracted and radioactivity was quantified by scintillation counting. Transgenic lines: aAGP-93 antisense expression of AGPase, line 93; AATP-62 overexpression of plastidic ATP/ADP transporter, line 62; aPGM-5 overexpression of phosphoglucomutase, line 5
ACCase activity is limiting for lipid synthesis in potato tubers
Acetyl-CoA and malonyl-CoA are the precursors for fatty acid synthesis in the plastids of plants. Malonyl-CoA, which is synthesized from acetyl-CoA by the acetyl-CoA carboxylase (ACCase) reaction, donates two carbons for each cycle of fatty acid chain elongation. For constitutive overexpression in potato, the ACCase gene from Arabidopsis was fused to a plastid targeting sequence and ligated into a binary vector behind the constitutive CaMV 35S promoter (Roesler et al. 1997). After transformation, three transgenic lines were selected based on Northern analysis of potato leaves (Fig. 3a). In the lines ACC-29 and ACC-91 a strong band at 7.5 kb was detected which corresponds to the size predicted from the ACCase open reading frame. In line ACC-2, a shorter band, presumably a truncated form of the transgenic ACCase mRNA, hybridized to Arabidopsis ACCase cDNA. To analyze protein expression in the different lines, total protein was isolated from WT and transformed potato tubers and used for immunoblot analysis with anti-biotin antibodies (Fig. 3b). A band of about 200 kDa was observed for lines ACC-29 and ACC-91, presumably representing Arabidopsis ACCase. In WT, a much weaker band derived from the endogenous, cytosolic potato protein was present. The Western blot was scanned to determine the relative expression level of the transgenic Arabidopsis ACCase in comparison to the endogenous potato protein. ACCase bands at 200 kDa were found to be increased by a factor of 2, 13 and 20 in the transgenic lines ACC-2, ACC-29 and ACC-91, respectively. Total ACCase activity was 8.7, 8.5, 96.1 and 85.8 pmol min−1 mg−1 protein in WT and the transgenic lines ACC-2, ACC-29 and ACC-91, respectively (Fig. 3c). Therefore, ACCase activity in the strong overexpressing lines ACC-29 and ACC-91 was increased by a factor of about 10 as compared to WT.
Overexpression of ACCase from Arabidopsis in transgenic potato. a Northern blot analysis of total leaf RNA isolated from WT and three ACCase-overexpressing lines. Top panel hybridization signal; lower panel ethidium bromide-stained rRNA bands. b Immunoblot of transgenic ACCase lines using anti-biotin antibodies. Total protein was isolated from potato tubers and after blotting, biotinylated proteins detected with anti-biotin antibodies. A band at around 200 kDa can be detected in the transgenic lines ACCase-29 and ACCase-91. c ACCase activity in protein extracts from tubers. Data represent mean ± SD of three measurements. d Triacylglycerol content in tubers. Lipids were quantified by GC of fatty acid methyl esters and data are presented as mean ± SD of three measurements. The inset shows fatty acid composition of triacylglycerol in WT and ACC-29 (in mol%, mean of three measurements; SD was less than 2 mol%)
The total fatty acid content on a fresh-weight basis was increased by about 30% in the two lines ACC-29 and ACC-91, from 1.08 to 1.39 and 1.35 mg g−1 FW, respectively (Table 2). Several lipid classes were elevated on a mg per g FW basis, i.e. total polar lipids, diacylglycerol and triacylglycerol, indicating that the additional fatty acids synthesized in the transgenic lines were channeled into different lipid pools. Quantification of polar lipids in WT and the transgenic lines demonstrated that there was no large change on a mol% basis for the major phospholipids (phosphatidylglycerol, phosphatidylinositol, phosphatidylethanolamine, phosphatidylcholine), for phosphatidic acid or for the galactolipids (monogalactosyldiacylglycerol, digalactosyldiacylglycerol; data not shown). Therefore, the absolute increase in total polar lipids was caused by a simultaneous increase in all polar lipids.
The increase in the amount of total fatty acids was accompanied by a decrease in α-linolenate (18:3) and a concomitant increase in linolate (18:2; data not shown). The change in the ratio of 18:2 to 18:3 might be explained by a limiting capacity for fatty acid desaturation under conditions of increased fatty acid synthesis. The relative amount of triacylglycerol increased from 1.2 mol% in WT to 4.6 mol% in the strongest overexpression line, ACC-29 (Table 2), indicating that a large fraction of fatty acids was preferentially re-directed to triacylglycerol synthesis. The ratio of dry weight to fresh weight in tubers was not changed in the three transgenic lines (21.8±2.0, 25.9±0.8, 20.8±0.6 and 21.1±2.1% for WT, ACC-2, ACC-29 and ACC-91, respectively; mean ± SD of four measurements). Therefore, lines ACC-29 and ACC-91 contained an even stronger increase in the amount of triacylglycerol when calculated per gram fresh weight or dry weight (0.0116 and 0.0580 mg g−1 FW for the WT and ACC-29, respectively; Fig. 3d). Similar to the total fatty acid composition, triacylglycerol contained elevated amounts of linolate (18:2) and reduced amounts of α-linolenate (18:3; Fig. 3d).
Fatty acid synthesis in WT and the three transgenic ACCase lines was measured by incorporation of [14C]acetate into lipids. The incorporation of radioactivity into lipids was calculated from the slopes after linear regression and was found to be 12, 13, 24 and 20 pmol mg−1 FW for WT, ACC-2, ACC-29 and ACC-91, respectively (Fig. 4). Therefore, flux from acetate into lipids as measured by in vitro labeling was increased by a factor of about two by ACCase overexpression.
Discussion
Photosynthates transported to the sink organs of plants can be metabolized and stored in the form of starch, oil or protein, or a mixture thereof. The proportions of these storage reserves vary greatly among plant species and determine to a large extent the food value and uses of crops. Therefore, understanding the mechanisms involved in carbon partitioning to plant storage reserves is a major goal with many biotechnological applications. Because many species are capable of synthesizing all of the different forms of storage compounds, it was hypothesized that a block in one of these pathways might result in a re-direction of carbon into one of the other storage forms. We tested two potato lines with alterations in starch synthesis (aAGP-93 and aPGMII-5) for their lipid content and fatty acid synthesis capacity. However, in neither of these lines was there an increase in total fatty acids or triacylglycerol when calculated on a fresh-weight basis. The ratio of dry weight to fresh weight was reduced in the line aAGP-93 (23.9±1.2 and 12.3±1.6% for WT and aAGP-93, respectively; mean ± SD, n=4; Müller-Röber et al. 1992). Therefore, the amount of total fatty acids (and thus, the amount of triacylglycerol) was increased in this line when calculated on a dry-weight basis (7.3±0.7 and 12.8±0.8 nmol total fatty acids mg−1 dry weight for WT and aAGP-93, respectively). Because the ratio of membrane to storage lipid was not altered in these lines (Table 1) and because the fatty acid synthesis activity was not increased (Fig. 2), the apparent increase in fatty acids per unit dry weight most likely is caused by the reduction of starch, rather than changes in lipid synthesis. Therefore, a block in starch synthesis does not result in the redirection of carbon into storage-lipid synthesis. The pea rugosus mutant contains reduced amounts of starch due to a block in starch-branching enzyme (Bhattacharyya et al. 1990). The seeds of this line are wrinkled and contain elevated amounts of lipid as calculated on a weight percent basis (Coxon and Davies 1982). Taking into account that the block in starch synthesis results in a reduction in seed weight, the change in lipid content is much less pronounced when calculated on a per seed basis. Therefore, it remains unclear whether in the pea rugosus mutant storage carbon is actively re-directed from starch to lipid.
The extra amount of carbon in transgenic potato lines blocked in starch synthesis accumulates in the form of soluble sugars or is degraded via glycolysis and the citrate cycle (Müller-Röber et al. 1992). To re-direct carbon into fatty acid and lipid synthesis, hexoses derived from sucrose need to be converted into acetyl-CoA via glycolysis. To increase lipid synthesis in potato tuber, it is therefore critical to determine rate-limiting steps in these pathways. Sucrose cleavage and conversion into hexose-phosphates occur at high rates in potato tubers as part of the sucrose-to-starch conversion. The enzymatic steps of glycolysis were shown to have activities between 135 nmol min−1 g−1 FW (phosphofructokinase) and 4,438 nmol min−1 g−1 FW (triose phosphate isomerase; Trethewey et al. 1998). In the course of this study, the activities of selected enzymes of lipid synthesis in potato tubers were determined as 11.2 pmol min−1 g−1 FW (ACCase), 0.073 pmol min−1 g−1 FW (fatty acid synthase) and 0.014 pmol min−1 g−1 FW (DGAT). Therefore, enzyme activities of lipid biosynthesis are at least 1,000-fold lower than those of glycolysis, suggesting that the reactions of lipid synthesis should be the primary targets of transgenic approaches to increase oil in potato tubers.
One possible factor limiting lipid synthesis is the amount of ATP, because ATP is required for malonyl-CoA production by ACCase in the plastid. An increase in plastidic ATP content resulted in an increased amount of starch in transgenic potato tubers (Tjaden et al. 1998; Geigenberger et al. 2001). However, the amount of triacylglycerol in line AATP-62 was not changed (Table 1). Therefore, the amount of plastidic ATP seems to be not limiting for fatty acid synthesis.
Because acylation of diacylglycerol is the only reaction unique to triacylglycerol synthesis, this enzyme was presumed to be crucial for the regulation of storage-lipid synthesis in plants. To test whether DGAT activity was limiting for storage-lipid production in potato tubers, the DGAT1 cDNA from Arabidopsis (derived from expressed sequence tag E6B2T7, GenBank accession AA042298) was overexpressed in potato under the control of the constitutive CaMV 35S promoter (binary vector pBINARHyg; von Schaeven 1989). Three transgenic lines were selected by Northern analysis of leaves showing strong overexpression of Arabidopsis DGAT1 mRNA (data not shown). However, in contrast to Arabidopsis seeds or tobacco leaves, overexpression of Arabidopsis DGAT1 in potato had no effect on the amount of total fatty acids or triacylglycerol in potato leaves or tubers (data not shown; Bouvier-Navé et al. 2000; Jako et al. 2001). In addition, lipid composition and fatty acid composition in leaves and tubers of transgenic potato lines overexpressing DGAT1 were unchanged. We measured DGAT activity by incubating transgenic potato leaf microsomes with dioleine and [1-14C]oleoyl-CoA as described by Jako et al. (2001). However, we could not observe any increase in triacylglycerol synthesis (data not shown). Sequencing of the Arabidopsis DGAT1 construct indicated no errors. It has been previously shown that DGAT activity and triacylglycerol synthesis in rape cell-suspension cultures can be stimulated by transfer to growth media with high sucrose concentration (Nykiforuk et al. 2002). The increase in DGAT activity in this system was at least in part attributed to posttranscriptional regulation. Therefore, the lack of increased DGAT activity in transgenic potato lines overexpressing Arabidopsis DGAT1 might be caused by posttranscriptional control of the Arabidopsis enzyme in the potato environment.
ACCase was found to be limiting for fatty acid synthesis in potato, because its overexpression in transgenic tubers led to a 30% increase in total fatty acid content in line ACC-29 (from 1.08 to 1.39 mg g−1 FW; Table 2) and a 5-fold increase in triacylglycerol (from 0.0116 to 0.0580 mg g−1 FW; Fig. 3d). Therefore, the additional amount of fatty acid was incorporated into all lipids, including membrane lipids, but was preferentially deposited as triacylglycerol. Potato tubers have a considerable capacity for lipid synthesis, and the activities of enzymes for triacylglycerol production, e.g. DGAT, are sufficient to incorporate the extra fatty acyl groups into triacylglycerol. Furthermore, the relative increase in total fatty acids or in triacylglycerol in the transgenic potato lines was much more pronounced as compared to the corresponding rapeseed lines (Roesler et al. 1997), where the total fatty acid content was increased by only 6% (384 mg g−1 and 408 mg g−1 dry weight for WT and transgenic ACCase rapeseed lines, respectively). This finding emphasizes the potential of using potato tubers as a model to study the impact of changes in enzyme activities on the accumulation of lipid or starch. However, the total amount and the triacylglycerol content in the strongest potato ACCase lines are still very low. Therefore, a more major re-direction of carbon from starch to oil will likely require a multi-target approach where different enzymatic or regulatory steps in carbohydrate and lipid synthesis are engineered.
Abbreviations
- AATP :
-
Plastidic ADP/ATP transporter
- ACCase :
-
Acetyl-CoA:carboxylase
- DGAT :
-
Acyl-CoA:diacylglycerol acyltransferase
- FW :
-
Fresh weight
- TLC :
-
Thin-layer chromatography
- WT:
-
Wild type
References
Bhattacharyya MK, Smith AM, Ellis THN, Hedley C, Martin C (1990) The wrinkled-seed character of pea described by Mendel is caused by a transposon-like insertion in a gene encoding starch-branching enzyme. Cell 60:115–122
Bouvier-Navé P, Benveniste P, Oelkers P, Sturley SL, Schaller H (2000) Expression in yeast and tobacco of plant cDNAs encoding acyl CoA:diacylglycerol acyltransferase. Eur J Biochem 267:85–96
Browse J, McCourt PJ, Somerville CR (1986) Fatty acid composition of leaf lipids determined after combined digestion and fatty acid methyl ester formation from fresh tissue. Anal Biochem 152:141–145
Coxon DT, Davies DR (1982) The effect of the ra and rb loci on the lipid content of the seed of Pisum sativum. Theor Appl Genet 64:47–50
Denyer K, Smith AM (1988) The capacity of plastids from developing pea cotyledons to synthesise acetyl CoA. Planta 173:172–182
Dörmann P, Hoffmann-Benning S, Balbo I, Benning C (1995) Isolation and characterization of an Arabidopsis mutant deficient in the thylakoid lipid digalactosyl diacylglycerol. Plant Cell 7:1801–1810
Focks N, Benning C (1998) wrinkled1: A novel, low-seed-oil mutant of Arabidopsis with a deficiency in the seed-specific regulation of carbohydrate metabolism. Plant Physiol 118:91–101
Galliard T (1968) Aspects of lipid metabolism in higher plants. I. Identification and quantitative determination of the lipids in potato tubers. Phytochemistry 7:1907–1914
Geigenberger P, Stamme C, Tjaden J, Schulz A, Quick PW, Betsche T, Kersting HJ, Neuhaus HE (2001) Tuber physiology and properties of starch from tubers of transgenic potato plants with altered plastidic adenylate transporter activity. Plant Physiol 125:16 67–1678
Jako C, Kumar A, Wei Y, Zou J, Barton DL, Giblin EM, Covello PS, Taylor DC (2001) Seed-specific over-expression of an Arabidopsis cDNA encoding a diacylglycerol acyltransferase enhances seed oil content and seed weight. Plant Physiol 126:861–874
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
Kang F, Rawsthorne S (1994) Starch and fatty acid synthesis in plastids from developing embryos of oilseed rape (Brassica napus L.) Plant J 6:795–805
Katavic V, Reed DW, Taylor DC, Giblin EM, Barton DL, Zou J, MacKenzie SL, Covello PS, Kunst L (1995) Alteration of seed fatty acid composition by an ethyl methanesulfonate-induced mutation in Arabidopsis thaliana affecting diacylglycerol acyltransferase activity. Plant Physiol 108:399–409
Liljenberg C, Sandelius AS, Selstam E (1978) Effect of storage in darkness and in light on the content of membrane lipids of potato tubers. Physiol Plant 43:154–159
Logemann J, Schell J, Willmitzer L (1987) Improved method for the isolation of RNA from plant tissues. Anal Biochem 163:16–20
McKeon TA, Stumpf PK (1982) Purification and characterization of the stearoyll-acyl carrier protein desaturase and the acyl-acyl carrier protein thioesterase from maturing seeds of safflower. J Biol Chem 257:12141–12147
Möhlmann T, Scheibe R, Neuhaus HE (1994) Interaction between fatty-acid and starch synthesis in isolated amyloplasts from cauliflower floral buds. Planta 194:492–497
Müller-Röber B, Sonnewald U, Willmitzer L (1992) Inhibition of the ADP-glucose pyrophosphorylase in transgenic potatoes leads to sugar-storing tubers and influences tuber formation and expression of tuber storage protein genes. EMBO J 11:1229–1238
Nykiforuk C, Furukawa-Stoffer TL, Huff PW, Sarna M, Laroche A, Moloney MM, Weselake RJ (2002) Characterization of cDNAs encoding diacylglycerol acyltransferase from cultures of Brassica napus and sucrose-mediated induction of enzyme biosynthesis. Biochim Biophys Acta 1580:95–109
Ohlrogge JB, Jaworski JG (1997) Regulation of fatty acid synthesis. Annu Rev Plant Physiol Plant Mol Biol 48:109–136
Palta JP, Whitaker BD, Weiss LS (1993) Plasma membrane lipids associated with genetic variability in freezing tolerance and cold acclimation of Solanum species. Plant Physiol 103:793–803
Pun WH, Khan AA, Chung I, Haydar M, Hadziyev D (1980) Lipid distribution in potato tubers. Potato Res 23:57–74
Rocha-Sosa M, Sonnewald U, Fommer W, Stratmann M, Schell J, Willmitzer L (1989) Both developmental and metabolic signals activate the promotor of a class I patatin gene. EMBO J 8:23–29
Roesler K, Shintani D, Savage L, Boddupalli S, Ohlrogge J (1997) Targeting of the Arabidopsis homomeric acetyl-coenzyme A carboxylase to plastids of rapeseeds. Plant Physiol 113:75–81
Roesler KR, Savage LJ, Shintani DK, Shorrosh BS, Ohlrogge JB (1996) Co-purification, co-immunoprecipitation and coordinate expression of acetyl-coenzyme A carboxylase activity, biotin carboxylase, and biotin carboxyl carrier protein of higher plants. Planta 198:517–525
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
Schaeven A von (1989) Untersuchungen zur ER vermittelten subzellulären Kompartimentierung fremder Proteine in höheren Pflanzen. PhD thesis, Free University Berlin, Germany
Schwender J, Ohlrogge JB (2002) Probing in vivo metabolism by stable isotope labeling of storage lipids and proteins in developing Brassica napus embryos. Plant Physiol 130:347–361
Spychalla JP (1994) Post-harvest potato tuber glycerolipid metabolism. Belknap WR, Vayda ME, Park WD (eds) The molecular and cellular biology of the potato, 2nd edn. CABI, Wallingford, UK
Tauberger E, Fernie A, Emmermann M, Renz A, Kossmann J, Willmitzer L, Trethewey RN (2000) Antisense inhibition of plastidial phosphoglucomutase provides compelling evidence that potato tuber amyloplast import carbon from the cytosol in the form of glucose-6-phosphate. Plant J 23:43–53
Trethewey RN, Geigenberger P, Riedel K, Hajirezaei M-R, Sonnewald U, Stitt M, Riesmeier JW, Willmitzer L (1998) Combined expression of glucokinase and invertase in potato tubers leads to a dramatic reduction in starch accumulation and a stimulation of glycolysis. Plant J 15:109–118
Tjaden J, Möhlmann T, Kampfenkel K, Henrichs G, Neuhaus HE (1998) Altered plastidic ATP/ADP-transporter activity influences potato (Solanum tuberosum L.) tuber morphology, yield and composition of tuber starch. Plant J 16:531–540
Zou J, Wei Y, Jako C, Kumar A, Selvaraj G, Taylor DC (1999) The Arabidopsis thaliana TAG1 mutant has a mutation in a diacylglycerol acyltransferase gene. Plant J 19:645–653
Acknowledgements
We thank E. Tauberger, B. Müller-Röber and A. Fernie (Max-Planck-Institute of Molecular Plant Physiologoy, Golm, Germany) for the transgenic potato lines altered in starch metabolism and Linda Savage (Department of Plant Biology, Michigan State University, East Lansing, USA) for performing Western blots of ACCase-overexpressing plants. This work was supported in part by a grant from the Deutsche Forschungsgemeinschaft (Grant Do 520/3-1; Bonn, Germany).
Author information
Authors and Affiliations
Corresponding author
Additional information
Source for transgenic plant material. Upon request, transgenic potato lines altered in ACCase activity can be obtained from Peter Dörmann. For potato lines with alterations in AATP transporter activity, please refer to H. Ekkehard Neuhaus. Transgenic AGP and PGM lines are available from A. Fernie (Max-Planck-Institute of Molecular Plant Physiology, Golm, Germany).
Rights and permissions
About this article
Cite this article
Klaus, D., Ohlrogge, J.B., Neuhaus, H.E. et al. Increased fatty acid production in potato by engineering of acetyl-CoA carboxylase. Planta 219, 389–396 (2004). https://doi.org/10.1007/s00425-004-1236-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-004-1236-3