Abstract
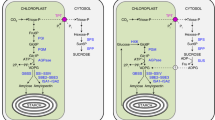
Transitory starch is stored during the day inside chloroplasts and then broken down at night for export. Recent data indicate that maltose is the major form of carbon exported from the chloroplast at night but its fate in the cytosol is unknown. An amylomaltase gene (malQ) cloned from Escherichia coli is necessary for maltose metabolism in E. coli. We investigated whether there is an amylomaltase in the cytosol of plant leaves and the role of this enzyme in plants. Two mutants of Arabidopsis thaliana (L) Heynh. were identified in which the gene encoding a putative amylomaltase enzyme [disproportionating enzyme 2, DPE2 (DPE1 refers to the plastid version of this enzyme)] was disrupted by a T-DNA insertion. Both dpe2-1 and dpe2-2 plants exhibited a dwarf phenotype and accumulated a large amount of maltose. In addition, dpe2 mutants accumulated starch and a water-soluble, ethanol/KCl-insoluble maltodextrin in their chloroplasts. At night, the amount of sucrose in dpe2 plants was lower than that in wild-type plants. These results show that Arabidopsis has an amylomaltase that is involved in the conversion of maltose to sucrose in the cytosol. We hypothesize that knocking out amylomaltase blocks the conversion from maltose to sucrose, and that the higher amount of maltose feeds back to limit starch degradation reactions in chloroplasts. As a result, dpe2 plants have higher maltose, higher starch, and higher maltodextrin but lower nighttime sucrose than wild-type plants. Finally, we propose that maltose metabolism in the cytosol of Arabidopsis leaves is similar to that in the cytoplasm of E. coli.








Similar content being viewed by others
Abbreviations
- F6P:
-
fructose 6-phosphate
- G1P:
-
glucose 1-phosphate
- G6P:
-
glucose 6-phosphate
- GTase:
-
glucanotransferase
References
Boos W, Shuman H (1998) Maltose/maltodextrin system of Escherichia coli: transport, metabolism, and regulation. Microbiol Mol Biol Rev 62:204–229
Critchley JH, Zeeman SC, Takaha T, Smith AM, Smith SM (2001) A critical role for disproportionating enzyme in starch breakdown is revealed by a knock-out mutation in Arabidopsis. Plant J 26:89–100
Duwenig E, Steup M, Willmitzer L, Kossmann J (1997) Antisense inhibition of cytosolic phosphorylase in potato plants (Solanum tubersosum L.) affects tuber sprouting and flower formation with only little impact on carbohydrate metabolism. Plant J 12:323–333
Emanuelsson O, Nielsen H, Brunak S, von Heijne G (2000) Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. J Mol Biol 300:1005–1016
Feinberg AP, Vogelstein B (1983) A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem 132:6–13
Goda SK, Eissa O, Akhtar M, Minton NP (1997) Molecular analysis of a Clostridium butyricum NCIMB 7423 gene encoding 4-alpha-glucanotransferase and characterization of the recombinant enzyme produced in Escherichia coli. Microbiology 143:3287–3294
Heldt HW, Chon CJ, Maronde D, Herold A, Stankovic ZS, Walker DA, Kraminer A, Kirk MR, Heber U (1977) Role of orthophosphate and other factors in the regulation of starch formation in leaves and isolated chloroplasts. Plant Physiol 59:1146–1155
Hofnung M, Schwartz M, Hatfield D (1971) Complementation studies in the maltose-A region of Escherichia coli K12 genetic map. J Mol Biol 61:681–694
Kakefuda G, Duke SH (1989) Characterization of pea chloroplast D-enzyme (4-α-glucanotransferase) Plant Physiol 91:136–143
Kakefuda G, Duke SH, Hostak MS (1986) Chloroplast and extrachloroplastic starch-degrading enzymes in Pisum sativum L. Planta 168:175–182
Kitahata S, Murakami H, Okada S (1989) Purification and some properties of amylomaltase from Escherichia coli IFO-3806. Agric Biol Chem 53:2653–2659
Lacks SA, Dunn JJ, Greenberg B (1982) Identification of base mismatches recognized by the heteroduplex-DNA-repair system of Streptococcus pneumoniae. Cell 31:327–336
Levi C, Gibbs M (1976) Starch degradation in isolated spinach chloroplasts. Plant Physiol 57:933–935
Lin T, Preiss J (1988) Characterisation of D-enzyme (4-α-glucanotransferase) in Arabidopsis leaf. Plant Physiol 86:260–265
Lowry OH, Passonneau JV (1972) A flexible system of enzymatic analysis. Academic Press, Orlando, FL, pp 1–291
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
Manners DJ, Rowe KL (1969) Studies on carbohydrate-metabolising enzymes: Part XXI. The α-1-glucosidase and D-enzyme activity of extracts of carrots and tomatoes. Carbohydr Res 9:441–450
Matsuura Y, Kusunoki M, Harada W, Kakudo M (1984) Structure and possible catalytic residues of taka-amylase a. J Biochem Tokyo 95:697–702
Mori H, Tanizawa K, Fuku T (1991) Potato tuber type H phosphorylase isozyme: molecular cloning, nucleotide sequence, and expression of a full-length cDNA in Escherichia coli. J Biol Chem 266:18446–18453
Okita TW, Greenberg E, Kuhn DN, Preiss J (1979) Subcellular localization of the starch degradative and biosynthetic enzymes of spinach leaves. Plant Physiol 64:187–192
Palmer NT, Ryman BE, Whelan WJ (1976) The action pattern of amylomaltase from Escherichia coli. Eur J Biochem 69:105–115
Peat S, Whelan WJ, Rees WR (1956) The enzymic synthesis and degradation of starch (part XX). The disproportionating enzyme (D-enzyme) of the potato. J Chem Soc 1956:44–53
Peavey DG, Steup M, Gibbs M (1977) Characterization of starch breakdown in the intact spinach chloroplast. Plant Physiol 60:305–308
Preiss J, Okita TW, Greenberg E (1980) Characterization of the spinach leaf phosphorylases. Plant Physiol 66:864–869
Pugsley AP, Dubrevil C (1988) Molecular characterization of malQ, the structural gene for the Escherichia coli enzyme amylomaltase. Mol Microbiol 2:473–479
Ritte G, Raschke K (2003) Metabolite export of isolated guard cell chloroplasts of Vicia faba. New Phytol 159:195–202
Schneider A, Häusler RE, Kolukisaoglu Ü, Kunze R, van der Graaff E, Schwacke R, Catoni E, Desimone M, Flügge U-I (2002) An Arabidopsis thaliana knock-out mutant of the chloroplast triose phosphate/phosphate translocator is severely compromised only when starch synthesis, but not starch mobilization is abolished. Plant J 32:685–699
Servaites JC, Geiger DR (2002) Kinetic characteristics of chloroplast glucose transport. J Exp Bot 53:1–11
Shirokane Y, Ichikawa K, Suzuki M (2000) A novel enzymic determination of maltose. Carbohydr Res 329:699–702
Steup M, Latzko E (1979) Intracellular localization of phosphorylases in spinach and pea leaves. Planta 145:69–75
Steup M, Schächtele C, Latzko E (1980) Purification of a non-chloroplastic α-glucan phosphorylase from spinach leaves. Planta 148:168–173
Stitt M, ap Rees T (1980) Carbohydrate breakdown by chloroplasts of Pisum sativum. Biochim Biophys Acta 627:131–143
Stitt M, Heldt HW (1981) Physiological rates of starch breakdown in isolated intact spinach chloroplasts. Plant Physiol 68:755–761
Stitt M, Quick WP (1989) Photosynthetic carbon partitioning: Its regulation and possibilities for manipulation. Physiol Plant 77:633–641
Szmelcman S, Schwartz M, Silhavy TJ, Boos W (1976) Maltose transport in Escherichia coli K12. A comparison of transport kinetics in wild-type and λ–resistant mutants with the dissociation constants of the maltose binding protein as measured by fluorescence quenching. Eur J Biochem 65:13–19
Takaha T, Smith SM (1999) The functions of 4-α-glucanotransferases and their use for the production of cyclic glucans. Biotech Gen Eng Rev 16:257–280
Takaha T, Yanase M, Okada S, Smith SM (1993) Disproportionating enzyme (4-α-glucanotransferase — EC 2.4.1.25) of potato — purification, molecular cloning, and potential role in starch metabolism. J Biol Chem 268:1391–1396
Takaha T, Critchley J, Okada S, Smith SM (1998) Normal starch content and composition in tubers of antisense potato plants lacking D-enzyme (4-α-glucanotransferase). Planta 205:445–451
Terada Y, Fujii K, Takaha T, Okada S (1999) Thermus aquaticus ATCC 33923 amylomaltase gene cloning and expression and enzyme characterization: production of cycloamylose. Appl Environ Microbiol 65:910–915
Trethewey RN, Smith AM (2000) Starch metabolism in leaves. In: Leegood RC, Sharkey TD, von Caemmerer S (eds) Photosynthesis: physiology and metabolism. Kluwer, Dordrecht, pp 205–231
Weigel D, Ahn JH, Blázquez MA, Borevitz JO, Christensen SK, Fankhauser C, Ferrándiz C, Kardailsky I, Malancharuvil EJ, Neff MM, Nguyen JT, Sato S, Wang Z-Y, Xia Y, Dixon RA, Harrison MJ, Lamb CJ, Yanofsky MF, Chory J (2000) Activation tagging in Arabidopsis. Plant Physiol 122:1003–1013
Weise SE, Weber APM, Sharkey TD (2003) Maltose is the major form of carbon exported from the chloroplast at night. Planta (in press)
Wiese A, Gröner F, Sonnewald U, Deppner H, Lerchl J, Hebbeker U, Flügge U-I, Weber A (1999) Spinach hexokinase I is located in the outer envelope membrane of plastids. FEBS Lett 461:13–18
Wintermans JFGM, DeMots A (1965) Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim Biophys Acta 109:448–453
Yang Y, Steup M (1990) Polysaccharide fraction from higher plants which strongly interacts with the cytosolic phosphorylase isozyme I. Isolation and characterization. Plant Physiol 94:960–969
Acknowledgements
This research was supported by the US Department of Energy under grant DE-FG02-99ER 20345. We thank the Biotech Center knockout facility of UW-Madison for access to T-DNA tagged plants. We also thank Donna E. Fernandez, Andreas P.M. Weber, and Sean E. Weise for their advice.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, Y., Sharkey, T.D. The role of amylomaltase in maltose metabolism in the cytosol of photosynthetic cells. Planta 218, 466–473 (2004). https://doi.org/10.1007/s00425-003-1127-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-003-1127-z