Abstract
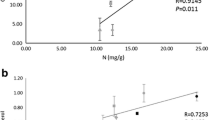
With the aim of understanding how some lichens can survive intensive fertilization we investigated two green algal (Trebouxia) lichens, Hypogymnia physodes (L.) Nyl. and Platismatia glauca (L.) W. Culb., and compared control (Ctr), and intensively fertilized (F) thalli. We measured total N, proteins and amino acids to assess lichen N status. Chlorophyll a indicated photosynthetic capacity and photobiont mass, ergosterol the metabolic demands of the fungus, and chitin the fungal biomass. For carbon status we measured glucose, the photobiont (Trebouxia) export product ribitol, and the mycobiont-specific carbohydrates arabitol and mannitol. The F-thalli had 2–3 times higher protein and N concentrations, 5–10 times higher chlorophyll a concentrations, while ergosterol and chitin were doubled. The ribitol concentrations were 4–5 times higher in the F-thalli, while the fungal carbohydrates did not increase to the same extent. The amino acid arginine had increased 60-fold. The F-thalli also had a relatively higher N investment in the photobiont in relation to mycobiont tissue compared to the Ctr-thalli, probably resulting in an increased capacity for carbon assimilation, most possibly required for maintaining the higher nutrient status of the F-thalli. Arginine accumulation possibly avoided toxic effects of accumulated NH4 +, albeit binding a significant fraction of assimilated carbon.






Similar content being viewed by others
Abbreviations
- aa:
-
total pool of amino acids
- aa−arg:
-
amino acids minus arginine
- arg:
-
arginine
- Chl a :
-
chlorophyll a
- Ctr-thalli:
-
control thalli collected from a non-fertilized control plot
- dw:
-
dry weight
- F-thalli:
-
fertilized thalli collected from an irrigation–fertilization (IL)-plot
- IL:
-
irrigation–fertilization designed to derive an optimal nutrient status in the Norway spruce needles (cf. Linder 1995)
References
Armstrong RA, Smith SN (1993) Radial growth and carbohydrate-levels in the lichen Parmelia conspersa on north and south facing rock surfaces. Symbiosis 15:27–38
Bergh J, Linder S, Lundmark T, Elfving B (1999) The effect of water and nutrient availability on the productivity of Norway spruce in northern and southern Sweden. For Ecol Manage 119:51–62
Beardall J, Griffiths H, Raven JA (1982) Carbon isotope discrimination and the CO2 accumulation mechanism in Chlorella emersonii. J Exp Bot 33:729–737
Boissière JC (1987) Ultrastructural relationship between the composition and the structure of the cell wall of the mycobiont of two lichens. Bibl Lichenol 25:117–123
Chapin III FS, Bloom AJ, Field CB, Waring RH (1987) Plant responses to multiple environmental factors. BioScience 37:49–57
Dahlman L, Zetherström M, Sundberg B, Näsholm T, Palmqvist K (2001) Measuring ergosterol and chitin in lichens. In: Kranner I, Beckett R, Varma A (eds) Protocols in lichenology—culturing, biochemistry, physiology and use in biomonitoring. Springer, Berlin, Heidelberg, New York, pp 348–362
Dahlman L, Näsholm T, Palmqvist K (2002) Growth, nitrogen uptake, and resource allocation in the two tripartite lichens Nephroma arcticum and Peltigera aphthosa during nitrogen stress. New Phytol 153:307–315
Davis RH (1984) Compartmental and regulatory mechanisms in the arginine pathways of Neurospora crassa and Saccharomyces cerevisiae. Microbiol Rev 50:280–313
Fahselt D (1994) Carbon metabolism in lichens. Symbiosis 17:127–182
Feige GB, Jensen M (1992) Basic carbon and nitrogen metabolism of lichens. In: Reisser W (ed) Algae and symbioses: plants, animals, fungi, viruses, interactions explored. Biopress, Bristol, pp 277–299
Gaio-Oliveira G, Branquinho C, Máguas C, Martins-Loucão A (2001) The concentration of nitrogen in nitrophilous and non-nitrophilous lichen species. Symbiosis 31:187–199
Hallingbäck T (1991) Blue-green algae and cyanophilic lichens are threatened by air pollution and fertilization (In Swedish with abstract in English). Sven Bot Tidskr 85:87–104
Honegger R (1991) Functional aspects of the lichen symbiosis Annu Rev Plant Physiol 42:553–578
Hyvärinen M, Crittenden PD (1998) Growth of the cushion-forming lichen, Cladonia portentosa at nitrogen-polluted and unpolluted heathland sites. Environ Exp Bot 40:67–76
Jensen MJ, Feige GB, Waterkotte A (1991) Mannitol-1-phosphate dehydrogenase in Psedovernia furfuracea. Lichenologist 23:187–196
Jäger H-J, Weigel H-J (1978) Amino acid metabolism in lichens. Bryologist 81:107–113
Katona ZF, Sass P, Molanár-Perl I (1999) Simultaneous determination of sugars, sugar alcohols, acids and amino acids in apricots by gas chromatography-mass spectrometry. J Chromatogr A 847:91–102
Lewis DH, Smith DC (1967) Sugar alcohols (polyols) in fungi and green plants. I. Distribution, physiology and metabolism. New Phytol 66:143–184
Linder S (1995) Foliar analysis for detecting and correcting nutrient imbalances in Norway spruce. Ecol Bull 44:178–190
Lines CEM, Ratcliffe RG, Rees TAV, Southon TE (1989) A 13C NMR study of photosynthate transport and metabolism in the lichen Xhantoria calicicola Oxner. New Phytol 111:447–456
Lövblad G, Andersen B, Hovmand M, Joffre S, Pedersen U, Reisell A (1992) Mapping deposition of sulphur, nitrogen and base cations in the Nordic countries. IVL Report B 1055. Swedish Enviromental Research Institute, Gothenburg
Nash TH Ⅲ (1996) Nutrients, elemental accumulation and mineral cycling. In: Nash TH Ⅲ (ed) Lichen biology. Cambridge University Press, Cambridge, pp 136–153
Näsholm T, Sandberg G, Ericsson A (1987) Quantitative analysis of amino acids in conifer tissues by high performance liquid chromatography and fluorescence detection of their 9-fluorenylmethyl chloroformate derivates. J Chromatogr A 396:225–236
Nordin A, Näsholm T (1997) Nitrogen storage forms in nine boreal understory plant species. Oecologia 110:487–492
Palmqvist K (2000) Carbon economy in lichens. New Phytol 148:11–36
Palmqvist K, Sundberg B (2001) Characterizing photosynthesis and respiration in freshly isolated or cultured lichen photobionts. In: Kranner I, Beckett R, Varma A (eds) Protocols in lichenology—culturing, biochemistry, physiology and use in biomonitoring. Springer, Berlin, Heidelberg, New York, pp 152–181
Palmqvist K, Badger M, Samuelsson MR (1994) Photobiont related differences in carbon acquisition among green-algal lichens. Planta 195:70–79
Palmqvist K, Campbell D, Ekblad A, Johansson H (1998) Photosynthetic capacity in relation to nitrogen content and its partitioning in lichens with different photobionts. Plant Cell Environ 21:361–372
Palmqvist K, Dahlman L, Valladares F, Tehler A, Sancho LG, Mattsson J-E (2002) CO2 exchange and thallus nitrogen across 75 contrasting lichen associations from different climate zones. Oecologia 133:295–306
Rabe E, Lovatt CJ (1986) Increased arginine-biosynthesis during phosphorus deficiency—a response to increased ammonia content of leaves. Plant Physiol 81:774–779
Rai AN (1988) Nitrogen metabolism. In: Galun M (ed) CRC handbook of lichenology, vol I. CRC Press, Boca Raton, pp 201–237
Raven JA (1991) Implications of organic carbon utilization: ecology, evolution and geochemistry. Can J Bot 69:908–924
Raven JA, Johnston AM, Handley LL, McInroy SG (1990) Transport and assimilation of inorganic carbon by Lichina pygmea under emersed and subemersed conditions. New Phytol 114:407–417
Richardson DHS (1999) War in the world of lichens: parasitism and symbiosis as exemplified by lichens and lichenicolous fungi. Mycol Res 6:641–650
Silberstein L, Siegel BZ, Siegel MS, Mukhtar A, Galun M (1996) Comparative studies on Xhantoria Parientina, a pollution-resistant lichen, and Ramalina duriaei, a sensitive species. 2. Evaluation of possible air pollution-protection mechanisms. Lichenologist 28:367–383
Smith EC, Griffiths H (1969) The occurrence of the chloroplast pyrenoid is correlated with the activity of a CO2 concentrating mechanism and carbon isotope discrimination in lichens and bryophytes. Planta 198:6–16
Smith SE, Read DJ (1997) Mycorrhizal symbiosis, 2nd edn. Academic Press, San Diego
Steinlein T, Heilmeier H, Schulze ED (1993) Nitrogen and carbohydrate storage in biennials originating from habitats of different resource availability. Oecologica 93:374–382
Sturgeon RJ (1985) Biosynthesis and utilization of storage sugars in algae, fungi, and lichens. Physiol Veg 23:95–106
Sundberg B, Ekblad A, Näsholm T, Palmqvist K (1999) Lichen respiration in relation to active time, nitrogen and ergosterol concentrations. Funct Ecol 13:119–125
Tretiach M, Pecchiari M (1995) Gas exchange rates and chlorophyll content of epi- and endolithic lichens from the Trieste Karst (NE Italy) New Phytol 130:585–592
Valladares F, Sancho LG, Ascaso C (1996) Functional analysis of the intrathalline and intracellular chlorophyll concentrations in the lichen family Umbilicariaceae. Ann Bot 78:471–477
Acknowledgements
The Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (FORMAS) provided grants to K.P. (24.0795/97) and T.N. (23.0345/99). The Center for Environmental Research (CMF, Umeå, Sweden) provided a grant to L.D. (993194). Margareta Zetherström (Department of Forest Genetics and Plant Physiology, SLU, Sweden) gave skilful technical support throughout. Professor Sune Linder (Department of Ecology and Environmental research, SLU, Sweden) is acknowledged for providing us with the unique lichen material resulting from the nutrient-optimization experiment at Flakaliden.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dahlman, L., Persson, J., Näsholm, T. et al. Carbon and nitrogen distribution in the green algal lichens Hypogymnia physodes and Platismatia glauca in relation to nutrient supply. Planta 217, 41–48 (2003). https://doi.org/10.1007/s00425-003-0977-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-003-0977-8