Abstract
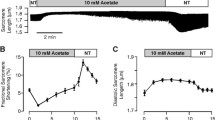
Dichloroacetate (DCA) and pyruvate activate pyruvate dehydrogenase (PDH), a key enzyme that modulates glucose oxidation and mitochondrial NADH production. Both compounds improve recovery after ischemia in isolated hearts. However, the action of DCA and pyruvate in normoxic myocardium is incompletely understood. We measured the effect of DCA and pyruvate on contraction, mitochondrial redox state, and intracellular calcium cycling in isolated rat hearts during normoxic perfusion. Normalized epicardial NADH fluorescence (nNADH) and left ventricular developed pressure (LVDP) were measured before and after administering DCA (5 mM) or pyruvate (5 mM). Optical mapping of Rhod-2AM was used to measure cytosolic calcium kinetics. DCA maximally activated PDH, increasing the ratio of active to total PDH from 0.48 ± 0.03 to 1.03 ± 0.03. Pyruvate sub-maximally activated PDH to a ratio of 0.75 ± 0.02. DCA and pyruvate increased LVDP. When glucose was the only exogenous fuel, pyruvate increased nNADH by 21.4 ± 2.9 % while DCA reduced nNADH by 21.4 ± 6.1 % and elevated the incidence of premature ventricular contractions (PVCs). When lactate, pyruvate, and glucose were provided together as exogenous fuels, nNADH increased with DCA, indicating that PDH activation with glucose as the only exogenous fuel depletes PDH substrate. Calcium transient time-to-peak was shortened by DCA and pyruvate and SR calcium re-uptake was 30 % longer. DCA and pyruvate increased SR calcium load in myocyte monolayers. Overall, during normoxia when glucose is the only exogenous fuel, DCA elevates SR calcium, increases LVDP and contractility, and diminishes mitochondrial NADH. Administering DCA with plasma levels of lactate and pyruvate mitigates the drop in mitochondrial NADH and prevents PVCs.







Similar content being viewed by others
References
Aasum E, Steigen T, Larsen T (1997) Stimulation of carbohydrate metabolism reduces hypothermia-induced calcium load in fatty acid-perfused rat hearts. J Mol Cell 534:527–534
Asfour H, Wengrowski AM, Jaimes R 3rd, Swift LM, Kay MW (2012) NADH fluorescence imaging of isolated biventricular working rabbit hearts. J Vis Exp 65:1–7
Ashruf JF, Coremans JM, Bruining HA, Ince C (1995) Increase of cardiac work is associated with decrease of mitochondrial NADH. Am J Physiol 269:H856–H862
Ashruf JF, Coremans JM, Bruining HA, Ince C (1996) Mitochondrial NADH in the Langendorff rat heart decreases in response to increases in work: increase of cardiac work is associated with decrease of mitochondrial NADH. Adv Exp Med Biol 388:275–282
Bassenge E, Sommer O, Schwemmer M, Bünger R (2000) Antioxidant pyruvate inhibits cardiac formation of reactive oxygen species through changes in redox state. Am J Physiol Heart Circ Physiol 279:H2431–H2438
Bersin RM, Stacpoole PW (1997) Dichloroacetate as metabolic therapy for myocardial ischemia and failure. Am Hear J 134:841–855
Blinova K, Levine RL, Boja ES, Griffiths GL, Shi Z-D, Ruddy B, Balaban RS (2008) Mitochondrial NADH fluorescence is enhanced by complex I binding. Biochemistry 47:9636–9645
Brandes R, Bers DM (1996) Increased work in cardiac trabeculae causes decreased mitochondrial NADH fluorescence followed by slow recovery. Biophys J 71:1024–1035
Bünger R, Mallet RT (1993) Mitochondrial pyruvate transport in working guinea-pig heart. Work-related vs. carrier-mediated control of pyruvate oxidation. Biochim Biophys Acta Biomembr 1151:223–236
Bünger R, Mallet RT, Hartman DA (1989) Pyruvate-enhanced phosphorylation potential and inotropism in normoxic and postischemic isolated working heart. Near-complete prevention of reperfusion contractile failure. Eur J Biochem 180:221–233
Chen W, London R, Murphy E, Steenbergen C (1998) Regulation of the Ca2+ gradient across the sarcoplasmic reticulum in perfused rabbit heart. A 19F nuclear magnetic resonance study. Circ Res 83:898–907
Combs C (2001) a, Balaban RS. Direct imaging of dehydrogenase activity within living cells using enzyme-dependent fluorescence recovery after photobleaching (ED-FRAP). Biophys J 80:2018–2028
De Hemptinne A, Marrannes R, Vanheel B (1983) Influence of organic acids on intracellular pH. Am J Physiol Cell Physiol 245:C178–C183
Fedorov VV, Lozinsky IT, Sosunov EA, Anyukhovsky EP, Rosen MR, Balke CW, Efimov IR (2007) Application of blebbistatin as an excitation-contraction uncoupler for electrophysiologic study of rat and rabbit hearts. Heart Rhythm 4:619–626
Gohil K, Jones DA (1983) A sensitive spectrophotometric assay for pyruvate dehydrogenase and oxoglutarate dehydrogenase complexes. Biosci Rep 3:1–9
Gore DC, Jahoor F, Hibbert JM, DeMaria EJ (1996) Lactic acidosis during sepsis is related to increased pyruvate production. Not deficits in tissue oxygen availability. Ann Surg 224:97–102
Gottlieb R, Magnus R (1904) Digitalis und Herzabeit. Nach Versuchen an uberlebenden Warmbluterherzen. Path Pharamakol 51:30–63
Hasenfuss G, Maier LS, Hermann H-P, Lüers C, Hünlich M, Zeitz O, Janssen PML, Pieske B (2002) Influence of pyruvate on contractile performance and Ca2+ cycling in isolated failing human myocardium. Circulation 105:194–199
Jackson VN, Halestrap AP (1996) The kinetics, substrate, and inhibitor specificity of the monocarboxylate (lactate) transporter of rat liver cells determined using the fluorescent intracellular pH indicator, 2’,7'-bis(carboxyethyl)-5(6)-carboxyfluorescein. J Biol Chem 271:861–868
Jin H, Nass RD, Joudrey PJ, Lyon AR, Chemaly ER, Rapti K, Akar FG (2010) Altered spatiotemporal dynamics of the mitochondrial membrane potential in the hypertrophied heart. Biophys J 98:2063–2071
Joubert F, Fales HM, Wen H, Combs CA, Balaban RS (2004) NADH enzyme-dependent fluorescence recovery after photobleaching (ED-FRAP): applications to enzyme and mitochondrial reaction kinetics, in vitro. Biophys J 86:629–645
Kang YH, Mallet RT, Bünger R (1992) Coronary autoregulation and purine release in normoxic heart at various cytoplasmic phosphorylation potentials: disparate effects of adenosine. Pflugers Arch 421:188–199
Kobayashi K, Neely JR (1983) Effects of ischemia and reperfusion on pyruvate dehydrogenase activity in isolated rat hearts. J Mol Cell Cardiol 15:359–367
Kuzmiak-Glancy S, Jaimes R, Wengrowski AM, Kay MW (2015) Oxygen demand of perfused heart preparations: how electromechanical function and inadequate oxygenation affect physiology and optical measurements. Exp Physiol. doi:10.1113/EP085042
Laughlin MR, Taylor J, Chesnick AS, DeGroot M, Balaban RS (1993) Pyruvate and lactate metabolism in the in vivo dog heart. Am J Physiol 264:H2068–H2079
Laurita KR, Katra R, Wible B, Wan X, Koo MH (2003) Transmural heterogeneity of calcium handling in canine. Circ Res 92:668–675
Lewandowski ED, Johnston DL (1990) Reduced substrate oxidation in postischemic myocardium: 13C and 31P NMR analyses. Am J Physiol Hear Circ Physiol 258:H1357–H1365
Lewandowski ED, White LT (1995) Pyruvate dehydrogenase influences postischemic heart function. Circulation 91:2071–2079
Liedtke AJ (1981) Alterations of carbohydrate and lipid metabolism in the acutely ischemic heart. Prog Cardiovasc Dis 23:321–336
Liu B, Clanachan AS, Schulz R, Lopaschuk GD (1996) Cardiac efficiency is improved after ischemia by altering both the source and fate of protons. Circ Res 79:940–948
Lloyd S, Brocks C, Chatham JC (2003) Differential modulation of glucose, lactate, and pyruvate oxidation by insulin and dichloroacetate in the rat heart. Am J Physiol Heart Circ Physiol 285:H163–H172
Lopaschuk GD, Wambolt RB, Barr RL (1993) An imbalance between glycolysis and glucose oxidation is a possible explanation for the detrimental effects of high levels of fatty acids during aerobic reperfusion of ischemic hearts. J Pharmacol Exp Ther 264:135–144
Mallet RT, Bunger R. Energetic modulation of cardiac inotropism and sarcoplasmic. Biochim Biophys Acta
Mallet R, Hartman D, Bunger R (1990) Glucose requirement for postischemic recovery of perfused working heart. Eur J Biochem 188:481–493
Mallet RT, Sun J (1999) Mitochondrial metabolism of pyruvate is required for its enhancement of cardiac function and energetics. Cardiovasc Res 42:149–161
Masoud WGT, Ussher JR, Wang W, Jaswal JS, Wagg CS, Dyck JR, Lygate CA, Neubauer S, Clanachan AS, Lopaschuk GD (2014) Failing mouse hearts utilize energy inefficiently and benefit from improved coupling of glycolysis and glucose oxidation. Cardiovasc Res 101:30–38
McVeigh JJ, Lopaschuk GD (1990) Dichloroacetate stimulation of glucose oxidation improves recovery of ischemic rat hearts. Am J Physiol 259:H1079–H1085
Nasa Y, Ichihara K, Abiko Y (1990) Myocardial non-esterified fatty acids during normoxia and ischemia in Langendorff and working rat hearts. Jpn J Pharmacol 53:129–133
Patel TB, Olson MS (1984) Regulation of pyruvate dehydrogenase complex in ischemic rat heart. Am J Physiol 246:H858–H864
Poole RC, Halestrap AP (1993) Transport of lactate and other monocarboxylates across mammalian plasma membranes. Am J Physiol 264:C761–C782
Posnack NG, Brooks D, Chandra A, Jaimes R 3rd, Sarvazyan N, Kay MW. Physiological response of cardiac tissue to Bisphenol A: alterations in ventricular pressure and contractility. Am J Physiol Heart Circ Physiol. doi: 10.1152/ajpheart.00272.2015
Posnack NG, Idrees R, Ding H, Jaimes R 3rd, Stybayeva G, Karabekian Z, Laflamme MA, Sarvazyan N (2015) Exposure to phthalates affects calcium handling and intercellular connectivity of human stem cell-derived cardiomyocytes. PLoS One 10, e0121927
Scholz TD, Laughlin MR, Balaban RS, Kupriyanov VV, Heineman FW (1995) Effect of substrate on mitochondrial NADH, cytosolic redox state, and phosphorylated compounds in isolated hearts. Am J Physiol 268:H82–H91
Schulze K, Duschek C, Lasley RD, Bünger R (2007) Adenosine enhances cytosolic phosphorylation potential and ventricular contractility in stunned guinea pig heart: receptor-mediated and metabolic protection. J Appl Physiol 102:1202–1213
Taniguchi M, Wilson C, Hunter CA, Pehowich DJ, Clanachan AS, Lopaschuk GD (2001) Dichloroacetate improves cardiac efficiency after ischemia independent of changes in mitochondrial proton leak. Am J Physiol Heart Circ Physiol 280:H1762–H1769
Torres CAA, Varian KD, Canan CH, Davis JP, Janssen PML (2013) The positive inotropic effect of pyruvate involves an increase in myofilament calcium sensitivity. PLoS One 8, e63608
Vincent G, Khairallah M, Bouchard B, Des RC (2003) Metabolic phenotyping of the diseased rat heart using 13C-substrates and ex vivo perfusion in the working mode. Mol Cell Biochem 242:89–99
Wahr JA, Olszanski D, Childs KF, Bolling SF (1996) Dichloroacetate enhanced myocardial functional recovery post-ischemia: ATP and NADH recovery. J Surg Res 63:220–224
Wengrowski AM, Kuzmiak-Glancy S, Jaimes R 3rd, Kay MW (2014) NADH changes during hypoxia, ischemia, and increased work differ between isolated heart preparations. Am J Physiol Heart Circ Physiol 306:H529–H537
White LT, O’Donnell JM, Griffin J, Lewandowski ED (1999) Cytosolic redox state mediates postischemic response to pyruvate dehydrogenase stimulation. Am J Physiol 277:H626–H634
Whitehouse S, Cooper RH, Randle PJ (1974) Mechanism of activation of pyruvate dehydrogenase by dichloroacetate and other halogenated carboxylic acids. Biochem J 141:761–774
Zima AV, Kockskämper J, Mejia-Alvarez R, Blatter LA (2003) Pyruvate modulates cardiac sarcoplasmic reticulum Ca2+ release in rats via mitochondria-dependent and -independent mechanisms. J Physiol 550:765–783
Acknowledgments
We acknowledge Dr. Jay H. Kramer and Dr. Brian Glancy for helpful discussions for determining PDH activity and Nate Serafino for technical assistance. This study was supported by NIH grant HL095828 (to MWK), NIH grant K99ES023477 (to NGP), and American Heart Association Postdoctoral Fellowship 14POST20490181 (to SKG).
Compliance with ethical standards
The authors declare that they have no conflict of interest. All applicable international, national, and institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the George Washington University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jaimes, R., Kuzmiak-Glancy, S., Brooks, D.M. et al. Functional response of the isolated, perfused normoxic heart to pyruvate dehydrogenase activation by dichloroacetate and pyruvate. Pflugers Arch - Eur J Physiol 468, 131–142 (2016). https://doi.org/10.1007/s00424-015-1717-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-015-1717-1