Abstract
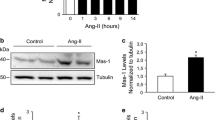
Angiotensin-(1–7) [Ang (1–7)] is a peptide belonging to the non-classical renin-angiotensin system (RAS). Ang (1–7), through its receptor Mas, has an opposite action to angiotensin II (Ang II), the typical peptide of the classical RAS axis. Ang II produces skeletal muscle atrophy, a pathological condition characterised by the loss of strength and muscle mass. A feature of muscle atrophy is the decrease of the myofibrillar proteins produced by the activation of the ubiquitin-proteasome pathway (UPP), evidenced by the increase in the expression of two muscle-specific ubiquitin ligases: atrogin-1 and MuRF-1. In addition, it has been described that Ang II also induces myonuclear apoptosis during muscle atrophy. We assessed the effects of Ang (1–7) and Mas participation on myonuclear apoptosis during skeletal muscle atrophy induced by Ang II. Our results show that Ang (1–7), through Mas, prevents the effects induced by Ang II in the diaphragm muscles and decreases several events associated with apoptosis in the diaphragm (increased apoptotic nuclei, increased expression of caspase-8 and caspase-9, increased caspase-3 activity and increased Bax/Bcl-2 ratio). Concomitantly, Ang (1–7) also attenuates the decrease in fibre diameter and muscle strength, and prevents the increase in atrogin-1 and MuRF-1 during the muscle wasting induced by Ang II. Interestingly, these effects of Ang (1–7) are dependent on the Mas receptor. Thus, we demonstrated for the first time that Ang (1–7) prevents myonuclear apoptosis during the recovery of skeletal muscle atrophy induced by Ang II.





Similar content being viewed by others
Abbreviations
- AngII:
-
Angiotensin II
- Ang (1–7):
-
Angiotensin 1–7
- ACE:
-
Angiotensin-converting enzyme
- RAS:
-
Renin-angiotensin system
- UPP:
-
Ubiquitin proteasome pathway
References
Acuna MJ, Pessina P, Olguin H, Cabrera D, Vio CP, Bader M, Munoz-Canoves P, Santos RA, Cabello-Verrugio C, Brandan E (2014) Restoration of muscle strength in dystrophic muscle by angiotensin 1–7 through inhibition of TGF-beta signalling. Hum Mol Genet 23(5):1237–1249. doi:10.1093/hmg/ddt514
Adhihetty PJ, O′Leary MF, Chabi B, Wicks KL (1985) Hood DA (2007) Effect of denervation on mitochondrially mediated apoptosis in skeletal muscle. J Appl Physiol 102(3):1143–1151. doi:10.1152/japplphysiol.00768.2006
Agarwal R (2003) Proinflammatory effects of oxidative stress in chronic kidney disease: role of additional angiotensin II blockade. Am J Physiol Ren Physiol 284(4):F863–869. doi:10.1152/ajprenal.00385.2002
Agarwal R, Campbell RC, Warnock DG (2004) Oxidative stress in hypertension and chronic kidney disease: role of angiotensin II. Semin Nephrol 24(2):101–114
Allen DL, Linderman JK, Roy RR, Bigbee AJ, Grindeland RE, Mukku V, Edgerton VR (1997) Apoptosis: a mechanism contributing to remodeling of skeletal muscle in response to hindlimb unweighting. Am J Physiol 273(2 Pt 1):C579–587
Antoniu SA (2008) Targeting the angiotensin pathway in idiopathic pulmonary fibrosis. Expert Opin Ther Targets 12(12):1587–1590. doi:10.1517/14728220802515459
Benter IF, Ferrario CM, Morris M, Diz DI (1995) Antihypertensive actions of angiotensin-(1–7) in spontaneously hypertensive rats. Am J Physiol 269(1 Pt 2):H313–319
Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ (2001) Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294(5547):1704–1708. doi:10.1126/science.1065874 1065874
Bonaldo P, Sandri M (2013) Cellular and molecular mechanisms of muscle atrophy. Dis Model Mech 6(1):25–39. doi:10.1242/dmm.010389
Brecher P (1996) Angiotensin II and cardiac fibrosis. Trends Cardiovasc Med 6(6):193–198. doi:10.1016/S1050-1738(96)00072–2
Brink M, Price SR, Chrast J, Bailey JL, Anwar A, Mitch WE, Delafontaine P (2001) Angiotensin II induces skeletal muscle wasting through enhanced protein degradation and down-regulates autocrine insulin-like growth factor I. Endocrinology 142(4):1489–1496
Brink M, Wellen J, Delafontaine P (1996) Angiotensin II causes weight loss and decreases circulating insulin-like growth factor I in rats through a pressor-independent mechanism. J Clin Invest 97(11):2509–2516. doi:10.1172/JCI118698
Cabello-Verrugio C, Acuna MJ, Morales MG, Becerra A, Simon F, Brandan E (2011) Fibrotic response induced by angiotensin-II requires NAD(P)H oxidase-induced reactive oxygen species (ROS) in skeletal muscle cells. Biochem Biophys Res Commun 410(3):665–670. doi:10.1016/j.bbrc.2011.06.051
Cabello-Verrugio C, Cordova G, Salas JD (2012) Angiotensin II: role in skeletal muscle atrophy. Curr Protein Pept Sci 13(6):560–569
Cabello-Verrugio C, Morales MG, Cabrera D, Vio CP, Brandan E (2012) Angiotensin II receptor type 1 blockade decreases CTGF/CCN2-mediated damage and fibrosis in normal and dystrophic skeletal muscles. J Cell Mol Med 16(4):752–764. doi:10.1111/j.1582-4934.2011.01354.x
Delafontaine P, Akao M (2006) Angiotensin II as candidate of cardiac cachexia. Curr Opin Clin Nutr Metab Care 9(3):220–224. doi:10.1097/01.mco.0000222103.29009.70
Dirks AJ, Leeuwenburgh C (2005) The role of apoptosis in age-related skeletal muscle atrophy. Sports Med 35(6):473–483
Echeverria-Rodriguez O, Del Valle-Mondragon L, Hong E (2013) Angiotensin 1–7 improves insulin sensitivity by increasing skeletal muscle glucose uptake in vivo. Peptides 51:26–30. doi:10.1016/j.peptides.2013.10.022
Eley HL, Skipworth RJ, Deans DA, Fearon KC, Tisdale MJ (2008) Increased expression of phosphorylated forms of RNA-dependent protein kinase and eukaryotic initiation factor 2alpha may signal skeletal muscle atrophy in weight-losing cancer patients. Br J Cancer 98(2):443–449. doi:10.1038/sj.bjc.6604150
Eley HL, Tisdale MJ (2007) Skeletal muscle atrophy, a link between depression of protein synthesis and increase in degradation. J Biol Chem 282(10):7087–7097. doi:10.1074/jbc.M610378200
Ferrario CM, Trask AJ, Jessup JA (2005) Advances in biochemical and functional roles of angiotensin-converting enzyme 2 and angiotensin-(1–7) in regulation of cardiovascular function. Am J Physiol Heart Circ Physiol 289(6):H2281–2290. doi:10.1152/ajpheart.00618.2005
Ferreira AJ, Santos RA, Raizada MK (2012) Angiotensin-(1–7)/angiotensin-converting enzyme 2/Mas receptor axis and related mechanisms. Int J Hypertens 2012:690785. doi:10.1155/2012/690785
Fidzianska A (2002) Suicide muscle cell programme-apoptosis. Ultrastructural study. Folia Neuropathol 40(1):27–32
Foletta VC, White LJ, Larsen AE, Leger B, Russell AP (2011) The role and regulation of MAFbx/atrogin-1 and MuRF1 in skeletal muscle atrophy. Pflugers Arch 461(3):325–335. doi:10.1007/s00424-010-0919-9
Glass DJ (2003) Molecular mechanisms modulating muscle mass. Trends Mol Med 9(8):344–350
Glass DJ (2010) Signaling pathways perturbing muscle mass. Curr Opin Clin Nutr Metab Care 13(3):225–229
Gomes MD, Lecker SH, Jagoe RT, Navon A, Goldberg AL (2001) Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci U S A 98(25):14440–14445. doi:10.1073/pnas.251541198
Gumucio JP, Mendias CL (2013) Atrogin-1, MuRF-1, and sarcopenia. Endocrine 43(1):12–21. doi:10.1007/s12020-012-9751-7
Hao Y, Jackson JR, Wang Y, Edens N, Pereira SL, Alway SE (2011) β-Hydroxy-β-methylbutyrate reduces myonuclear apoptosis during recovery from hind limb suspension-induced muscle fiber atrophy in aged rats. Am J Physiol Regul Integr Comp Physiol 301(3):R701–715. doi:10.1152/ajpregu.00840.2010
Henriksen EJ, Prasannarong M (2012) The role of the renin-angiotensin system in the development of insulin resistance in skeletal muscle. Mol Cell Endocrinol 378(1–2):15–22. doi:10.1016/j.mce.2012.04.011
Iwata M, Cowling RT, Gurantz D, Moore C, Zhang S, Yuan JX, Greenberg BH (2005) Angiotensin-(1–7) binds to specific receptors on cardiac fibroblasts to initiate antifibrotic and antitrophic effects. Am J Physiol Heart Circ Physiol 289(6):H2356–2363. doi:10.1152/ajpheart.00317.2005
Jiang T, Gao L, Shi J, Lu J, Wang Y, Zhang Y (2013) Angiotensin-(1–7) modulates renin-angiotensin system associated with reducing oxidative stress and attenuating neuronal apoptosis in the brain of hypertensive rats. Pharmacol Res 67(1):84–93. doi:10.1016/j.phrs.2012.10.014
Kackstein K, Teren A, Matsumoto Y, Mangner N, Mobius-Winkler S, Linke A, Schuler G, Punkt K, Adams V (2012) Impact of angiotensin II on skeletal muscle metabolism and function in mice: contribution of IGF-1, Sirtuin-1 and PGC-1alpha. Acta Histochem 115:363–370. doi:10.1016/j.acthis.2012.09.009
Libera LD, Zennaro R, Sandri M, Ambrosio GB, Vescovo G (1999) Apoptosis and atrophy in rat slow skeletal muscles in chronic heart failure. Am J Physiol 277(5 Pt 1):C982–986
Lokireddy S, Mouly V, Butler-Browne G, Gluckman PD, Sharma M, Kambadur R, McFarlane C (2011) Myostatin promotes the wasting of human myoblast cultures through promoting ubiquitin-proteasome pathway-mediated loss of sarcomeric proteins. Am J Physiol Cell Physiol 301(6):C1316–1324. doi:10.1152/ajpcell.00114.2011
Mancini GB, Khalil N (2005) Angiotensin II type 1 receptor blocker inhibits pulmonary injury. Clin Invest Med 28(3):118–126
Marangoni RA, Carmona AK, Passaglia RC, Nigro D, Fortes ZB, de Carvalho MH (2006) Role of the kallikrein-kinin system in Ang-(1–7)-induced vasodilation in mesenteric arterioles of Wistar rats studied in vivo-in situ. Peptides 27(7):1770–1775. doi:10.1016/j.peptides.2006.02.002
Marzetti E, Calvani R, Bernabei R, Leeuwenburgh C (2011) Apoptosis in skeletal myocytes: a potential target for interventions against sarcopenia and physical frailty—a mini-review. Gerontology 58(2):99–106. doi:10.1159/000330064
Marzetti E, Groban L, Wohlgemuth SE, Lees HA, Lin M, Jobe H, Giovannini S, Leeuwenburgh C, Carter CS (2008) Effects of short-term GH supplementation and treadmill exercise training on physical performance and skeletal muscle apoptosis in old rats. Am J Physiol Regul Integr Comp Physiol 294(2):R558–567. doi:10.1152/ajpregu.00620.2007
Marzetti E, Lawler JM, Hiona A, Manini T, Seo AY, Leeuwenburgh C (2008) Modulation of age-induced apoptotic signaling and cellular remodeling by exercise and calorie restriction in skeletal muscle. Free Radic Biol Med 44(2):160–168. doi:10.1016/j.freeradbiomed.2007.05.028
Marzetti E, Privitera G, Simili V, Wohlgemuth SE, Aulisa L, Pahor M, Leeuwenburgh C (2010) Multiple pathways to the same end: mechanisms of myonuclear apoptosis in sarcopenia of aging. ScientificWorldJournal 10:340–349. doi:10.1100/tsw.2010.27
Masson S, Latini R, Bevilacqua M, Vago T, Sessa F, Torri M, Anesini A, Salio M, Pasotti E, Agnello D, Santoro L, Catania A, Ghezzi P, Moccetti T, Maggioni AP (1998) Within-patient variability of hormone and cytokine concentrations in heart failure. Pharmacol Res 37(3):213–217. doi:10.1006/phrs.1998.0288
Morales MG, Abrigo J, Meneses C, Simon F, Cisternas F, Rivera JC, Vazquez Y, Cabello-Verrugio C (2014) Angiotensin 1-7/Mas-1 axis attenuates the expression and signaling of TGF-beta1 induced by Angiotensin II in skeletal muscle. Clin Sci (Lond). doi:10.1042/CS20130585
Morales MG, Cabello-Verrugio C, Santander C, Cabrera D, Goldschmeding R, Brandan E (2011) CTGF/CCN-2 over-expression can directly induce features of skeletal muscle dystrophy. J Pathol 225(4):490–501. doi:10.1002/path.2952
Morales MG, Cabrera D, Cespedes C, Vio CP, Vazquez Y, Brandan E, Cabello-Verrugio C (2013) Inhibition of the angiotensin-converting enzyme decreases skeletal muscle fibrosis in dystrophic mice by a diminution in the expression and activity of connective tissue growth factor (CTGF/CCN-2). Cell Tissue Res 353(1):173–187. doi:10.1007/s00441-013-1642-6
Morales MG, Vazquez Y, Acuna MJ, Rivera JC, Simon F, Salas JD, Alvarez Ruf J, Brandan E, Cabello-Verrugio C (2012) Angiotensin II-induced pro-fibrotic effects require p38MAPK activity and transforming growth factor beta 1 expression in skeletal muscle cells. Int J Biochem Cell Biol 44(11):1993–2002. doi:10.1016/j.biocel.2012.07.028
Munoz MC, Giani JF, Burghi V, Mayer MA, Carranza A, Taira CA, Dominici FP (2012) The Mas receptor mediates modulation of insulin signaling by angiotensin-(1–7). Regul Pept 177(1–3):1–11. doi:10.1016/j.regpep.2012.04.001
Painemal P, Acuna MJ, Riquelme C, Brandan E, Cabello-Verrugio C (2013) Transforming growth factor type beta 1 increases the expression of angiotensin II receptor type 2 by a SMAD- and p38 MAPK-dependent mechanism in skeletal muscle. Biofactors 39(4):467–475. doi:10.1002/biof.1087
Powers SK, Kavazis AN, McClung JM (2007) Oxidative stress and disuse muscle atrophy. J Appl Physiol 102(6):2389–2397. doi:10.1152/japplphysiol.01202.2006
Prasannarong M, Santos FR, Henriksen EJ (2012) ANG-(1–7) reduces ANG II-induced insulin resistance by enhancing Akt phosphorylation via a Mas receptor-dependent mechanism in rat skeletal muscle. Biochem Biophys Res Commun 426(3):369–373. doi:10.1016/j.bbrc.2012.08.093
Primeau AJ, Adhihetty PJ, Hood DA (2002) Apoptosis in heart and skeletal muscle. Can J Appl Physiol 27(4):349–395
Rezk BM, Yoshida T, Semprun-Prieto L, Higashi Y, Sukhanov S, Delafontaine P (2012) Angiotensin II infusion induces marked diaphragmatic skeletal muscle atrophy. PLoS One 7(1):e30276. doi:10.1371/journal.pone.0030276
Roig E, Perez-Villa F, Morales M, Jimenez W, Orus J, Heras M, Sanz G (2000) Clinical implications of increased plasma angiotensin II despite ACE inhibitor therapy in patients with congestive heart failure. Eur Heart J 21(1):53–57. doi:10.1053/euhj.1999.1740
Russell ST, Eley H, Tisdale MJ (2007) Role of reactive oxygen species in protein degradation in murine myotubes induced by proteolysis-inducing factor and angiotensin II. Cell Signal 19(8):1797–1806. doi:10.1016/j.cellsig.2007.04.003
Russell ST, Wyke SM, Tisdale MJ (2006) Mechanism of induction of muscle protein degradation by angiotensin II. Cell Signal 18(7):1087–1096. doi:10.1016/j.cellsig.2005.09.009
Sabharwal R, Chapleau MW (2014) Autonomic, locomotor and cardiac abnormalities in a mouse model of muscular dystrophy: targeting the renin-angiotensin system. Exp Physiol 99(4):627–631. doi:10.1113/expphysiol.2013.074336
Sabharwal R, Cicha MZ, Sinisterra RD, De Sousa FB, Santos RA, Chapleau MW (2014) Chronic oral administration of Ang-(1–7) improves skeletal muscle, autonomic and locomotor phenotypes in muscular dystrophy. Clin Sci (Lond) 127(2):101–109. doi:10.1042/CS20130602
Sanders PM, Russell ST, Tisdale MJ (2005) Angiotensin II directly induces muscle protein catabolism through the ubiquitin-proteasome proteolytic pathway and may play a role in cancer cachexia. Br J Cancer 93(4):425–434. doi:10.1038/sj.bjc.6602725
Sandri M (2008) Signaling in muscle atrophy and hypertrophy. Physiology (Bethesda) 23:160–170. doi:10.1152/physiol.00041.2007
Santos RA, Simoes E, Silva AC, Maric C, Silva DM, Machado RP, de Buhr I, Heringer-Walther S, Pinheiro SV, Lopes MT, Bader M, Mendes EP, Lemos VS, Campagnole-Santos MJ, Schultheiss HP, Speth R, Walther T (2003) Angiotensin-(1–7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci U S A 100(14):8258–8263. doi:10.1073/pnas.1432869100
Schiaffino S, Dyar KA, Ciciliot S, Blaauw B, Sandri M (2013) Mechanisms regulating skeletal muscle growth and atrophy. FEBS J 280(17):4294–4314. doi:10.1111/febs.12253
Semprun-Prieto LC, Sukhanov S, Yoshida T, Rezk BM, Gonzalez-Villalobos RA, Vaughn C, Michael Tabony A, Delafontaine P (2011) Angiotensin II induced catabolic effect and muscle atrophy are redox dependent. Biochem Biophys Res Commun 409(2):217–221. doi:10.1016/j.bbrc.2011.04.122
Shenkman BS, Turtikova OV, Nemirovskaya TL, Grigoriev AI (2010) Skeletal muscle activity and the fate of myonuclei. Acta Nat 2(2):59–66
Simic G, Seso-Simic D, Lucassen PJ, Islam A, Krsnik Z, Cviko A, Jelasic D, Barisic N, Winblad B, Kostovic I, Kruslin B (2000) Ultrastructural analysis and TUNEL demonstrate motor neuron apoptosis in Werdnig-Hoffmann disease. J Neuropathol Exp Neurol 59(5):398–407
Siu PM, Alway SE (2005) Mitochondria-associated apoptotic signalling in denervated rat skeletal muscle. J Physiol 565(Pt 1):309–323. doi:10.1113/jphysiol.2004.081083
Siu PM, Pistilli EE, Alway SE (2005) Apoptotic responses to hindlimb suspension in gastrocnemius muscles from young adult and aged rats. Am J Physiol Regul Integr Comp Physiol 289(4):R1015–1026. doi:10.1152/ajpregu.00198.2005
Song YH, Li Y, Du J, Mitch WE, Rosenthal N, Delafontaine P (2005) Muscle-specific expression of IGF-1 blocks angiotensin II-induced skeletal muscle wasting. J Clin Invest 115(2):451–458. doi:10.1172/JCI22324
Sudo M, Kano Y (2009) Myofiber apoptosis occurs in the inflammation and regeneration phase following eccentric contractions in rats. J Physiol Sci 59(6):405–412. doi:10.1007/s12576-009-0049-3
Sukhanov S, Semprun-Prieto L, Yoshida T, Michael Tabony A, Higashi Y, Galvez S, Delafontaine P (2011) Angiotensin II, oxidative stress and skeletal muscle wasting. Am J Med Sci 342(2):143–147. doi:10.1097/MAJ.0b013e318222e620
Tallant EA, Ferrario CM, Gallagher PE (2005) Angiotensin-(1–7) inhibits growth of cardiac myocytes through activation of the Mas receptor. Am J Physiol Heart Circ Physiol 289(4):H1560–1566. doi:10.1152/ajpheart.00941.2004
Yoshida T, Semprun-Prieto L, Sukhanov S, Delafontaine P (2010) IGF-1 prevents ANG II-induced skeletal muscle atrophy via Akt- and Foxo-dependent inhibition of the ubiquitin ligase atrogin-1 expression. Am J Physiol Heart Circ Physiol 298(5):H1565–1570. doi:10.1152/ajpheart.00146.2010
Acknowledgments
The authors thank Darling Vera for the technical assistance.
Funding
This study was supported by research grants from Association-Francaise Contre Les Myopathies AFM 16670 (CCV); FONDECYT 1120380 (CCV), 3130593 (MGM), 1121078 (FS), 1110426 (EB); Millennium Institute on Immunology and Immunotherapy, P09-016-F (FS); CARE PFB12/2007 (EB), Fundación Chilena para Biología Celular Proyecto MF-100; UNAB-DI-281-13/R (CCV).
Conflict of interest
The authors confirm that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
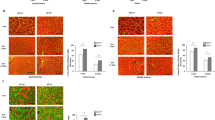
Angiotensin-(1–7) reverts the decrease of fiber diameter in the diaphragm during muscle wasting induced by angiotensin II. C57BL10 mice were systemically treated with the vehicle (a), Ang II (b), Ang (1–7) (c), Ang II + Ang (1–7) (d), A779 (e), or Ang II + Ang (1–7) + A779 (f) for 14 days as described in the Materials and Methods section. Haematoxylin and eosin staining was performed on cryosections of the diaphragm. The images are representative of three independent experiments, using four mice for each experimental condition. The bar corresponds to 50 μm. (GIF 673 kb)
Rights and permissions
About this article
Cite this article
Meneses, C., Morales, M.G., Abrigo, J. et al. The angiotensin-(1–7)/Mas axis reduces myonuclear apoptosis during recovery from angiotensin II-induced skeletal muscle atrophy in mice. Pflugers Arch - Eur J Physiol 467, 1975–1984 (2015). https://doi.org/10.1007/s00424-014-1617-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-014-1617-9