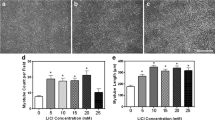
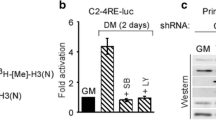
Abstract
Myoblast differentiation is mediated by a cascade of changes in gene expression including transcription factors such as myogenin. Subsequent to myoblast differentiation, there is an increase in expression of the transmembrane protein NADPH oxidase (Nox). Nox is one of the primary factors for the generation of reactive oxygen species (ROS) in myogenic (C2C12) cells. Recently, ROS have been shown to be important regulators of several intracellular signaling pathways, and the full extent of their regulatory roles is yet to be discovered. In the present study, qRT PCR analysis demonstrated that Nox4 isoform is primarily expressed in differentiating C2C12 cells and contributes to the generation of ROS in C2C12 myoblast during differentiation. Over-expression and silencing of Nox4 expression during myoblast differentiation was accompanied by a reduction in intracellular ROS concentrations and an alteration in the expression patterns of Myf5, Pax7, MyoD1, and myogenin. This modulation was found to be associated with ERK1/2 phosphorylation. In both over-expression and reduced expression of Nox4, we found significant reductions in ERK1/2 phosphorylation. This indicates that cellular differentiation may be affected by Nox4-mediated endogenous ROS generation. These data suggest a new opportunity to study the temporal expression of Nox4 in the generation of ROS accompanying changes in myogenic differentiation.








Similar content being viewed by others
References
Aguiari P, Leo S, Zavan B, Vindigni V, Rimessi A, Bianchi K, Franzin C, Cortivo R, Rossato M, Vettor R, Abatangelo G, Pozzan T, Pinton P, Rizzuto R (2008) High glucose induces adipogenic differentiation of muscle-derived stem cells. Proc Natl Acad Sci U S A 105(4):1226–1231. doi:10.1073/pnas.0711402105
Andres V, Walsh K (1996) Myogenin expression, cell cycle withdrawal, and phenotypic differentiation are temporally separable events that precede cell fusion upon myogenesis. J Cell Biol 132(4):657–666
Bedard K, Krause KH (2007) The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87(1):245–313
Berkes CA, Bergstrom DA, Penn BH, Seaver KJ, Knoepfler PS, Tapscott SJ (2004) Pbx marks genes for activation by MyoD indicating a role for a homeodomain protein in establishing myogenic potential. Mol Cell 14(4):465–477
Brennan TJ, Olson EN (1990) Myogenin resides in the nucleus and acquires high affinity for a conserved enhancer element on heterodimerization. Genes Dev 4(4):582–595
Breusing N, Grune T (2008) Regulation of proteasome-mediated protein degradation during oxidative stress and aging. Biol Chem 389(3):203–209. doi:10.1515/BC.2008.029
Cabello-Verrugio C, Acuna MJ, Morales MG, Becerra A, Simon F, Brandan E (2011) Fibrotic response induced by angiotensin-II requires NAD(P)H oxidase-induced reactive oxygen species (ROS) in skeletal muscle cells. Biochem Biophys Res Commun 410(3):665–670. doi:10.1016/j.bbrc.2011.06.051
Carnac G, Primig M, Kitzmann M, Chafey P, Tuil D, Lamb N, Fernandez A (1998) RhoA GTPase and serum response factor control selectively the expression of MyoD without affecting Myf5 in mouse myoblasts. Mol Biol Cell 9(7):1891–1902
Cheguru P, Chapalamadugu KC, Doumit ME, Murdoch GK, Hill RA (2012) Adipocyte differentiation-specific gene transcriptional response to C18 unsaturated fatty acids plus insulin. Pflugers Arch 463(3):429–447. doi:10.1007/s00424-011-1066-7
Chen Y, Azad MB, Gibson SB (2009) Superoxide is the major reactive oxygen species regulating autophagy. Cell Death Differ 16(7):1040–1052. doi:10.1038/cdd.2009.49
Choi H, Kim S, Kim HJ, Kim KM, Lee CH, Shin JH, Noh M (2010) Sphingosylphosphorylcholine down-regulates filaggrin gene transcription through NOX5-based NADPH oxidase and cyclooxygenase-2 in human keratinocytes. Biochem Pharmacol 80(1):95–103. doi:10.1016/j.bcp.2010.03.009
Clemente CF, Corat MA, Saad ST, Franchini KG (2005) Differentiation of C2C12 myoblasts is critically regulated by FAK signaling. Am J Physiol Regul Integr Comp Physiol 289(3):R862–R870. doi:10.1152/ajpregu.00348.2004
Collins CA, Gnocchi VF, White RB, Boldrin L, Perez-Ruiz A, Relaix F, Morgan JE, Zammit PS (2009) Integrated functions of Pax3 and Pax7 in the regulation of proliferation, cell size and myogenic differentiation. PLoS One 4(2):e4475. doi:10.1371/journal.pone.0004475
Dedieu S, Mazeres G, Cottin P, Brustis JJ (2002) Involvement of myogenic regulator factors during fusion in the cell line C2C12. Int J Dev Biol 46(2):235–241
Desouki MM, Kulawiec M, Bansal S, Das GM, Singh KK (2005) Cross talk between mitochondria and superoxide generating NADPH oxidase in breast and ovarian tumors. Cancer Biol Ther 4(12):1367–1373
Diel P, Baadners D, Schlupmann K, Velders M, Schwarz JP (2008) C2C12 myoblastoma cell differentiation and proliferation is stimulated by androgens and associated with a modulation of myostatin and Pax7 expression. J Mol Endocrinol 40(5):231–241. doi:10.1677/JME-07-0175
Ding Y, Choi KJ, Kim JH, Han X, Piao Y, Jeong JH, Choe W, Kang I, Ha J, Forman HJ, Lee J, Yoon KS, Kim SS (2008) Endogenous hydrogen peroxide regulates glutathione redox via nuclear factor erythroid 2-related factor 2 downstream of phosphatidylinositol 3-kinase during muscle differentiation. Am J Pathol 172(6):1529–1541. doi:10.2353/ajpath.2008.070429
Geiszt M (2006) NADPH oxidases: new kids on the block. Cardiovasc Res 71(2):289–299. doi:10.1016/j.cardiores.2006.05.004
Goettsch C, Goettsch W, Muller G, Seebach J, Schnittler HJ, Morawietz H (2009) Nox4 overexpression activates reactive oxygen species and p38 MAPK in human endothelial cells. Biochem Biophys Res Commun 380(2):355–360
Goyal P, Weissmann N, Grimminger F, Hegel C, Bader L, Rose F, Fink L, Ghofrani HA, Schermuly RT, Schmidt HH, Seeger W, Hanze J (2004) Upregulation of NAD(P)H oxidase 1 in hypoxia activates hypoxia-inducible factor 1 via increase in reactive oxygen species. Free Radic Biol Med 36(10):1279–1288. doi:10.1016/j.freeradbiomed.2004.02.071S0891584904001893
Hancock JT, Desikan R, Neill SJ (2001) Role of reactive oxygen species in cell signalling pathways. Biochem Soc Trans 29(Pt 2):345–350
Handayaningsih AE, Iguchi G, Fukuoka H, Nishizawa H, Takahashi M, Yamamoto M, Herningtyas EH, Okimura Y, Kaji H, Chihara K, Seino S, Takahashi Y (2011) Reactive oxygen species play an essential role in IGF-I signaling and IGF-I-induced myocyte hypertrophy in C2C12 myocytes. Endocrinology 152(3):912–921. doi:10.1210/en.2010-0981
Hecker L, Vittal R, Jones T, Jagirdar R, Luckhardt TR, Horowitz JC, Pennathur S, Martinez FJ, Thannickal VJ (2009) NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med 15(9):1077–1081. doi:10.1038/nm.2005
Hilenski LL, Clempus RE, Quinn MT, Lambeth JD, Griendling KK (2004) Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 24(4):677–683. doi:10.1161/01.ATV.0000112024.13727.2c
Horst D, Ustanina S, Sergi C, Mikuz G, Juergens H, Braun T, Vorobyov E (2006) Comparative expression analysis of Pax3 and Pax7 during mouse myogenesis. Int J Dev Biol 50(1):47–54. doi:10.1387/ijdb.052111dh
Hutchinson DS, Csikasz RI, Yamamoto DL, Shabalina IG, Wikstrom P, Wilcke M, Bengtsson T (2007) Diphenylene iodonium stimulates glucose uptake in skeletal muscle cells through mitochondrial complex I inhibition and activation of AMP-activated protein kinase. Cell Signal 19(7):1610–1620. doi:10.1016/j.cellsig.2007.02.006
Jones NC, Fedorov YV, Rosenthal RS, Olwin BB (2001) ERK1/2 is required for myoblast proliferation but is dispensable for muscle gene expression and cell fusion. J Cell Physiol 186(1):104–115. doi:10.1002/1097-4652(200101)186:1 < 104::AID-JCP1015 > 3.0.CO;2-0
Kim KS, Choi HW, Yoon HE, Kim IY (2010) Reactive oxygen species generated by NADPH oxidase 2 and 4 are required for chondrogenic differentiation. J Biol Chem 285(51):40294–40302. doi:10.1074/jbc.M110.126821
Kim BW, Lee JW, Choo HJ, Lee CS, Jung SY, Yi JS, Ham YM, Lee JH, Hong J, Kang MJ, Chi SG, Hyung SW, Lee SW, Kim HM, Cho BR, Min DS, Yoon G, Ko YG (2010) Mitochondrial oxidative phosphorylation system is recruited to detergent-resistant lipid rafts during myogenesis. Proteomics 10(13):2498–2515. doi:10.1002/pmic.200900826
Kook SH, Lee HJ, Chung WT, Hwang IH, Lee SA, Kim BS, Lee JC (2008) Cyclic mechanical stretch stimulates the proliferation of C2C12 myoblasts and inhibits their differentiation via prolonged activation of p38 MAPK. Mol Cells 25(4):479–486
Kuroda J, Ago T, Matsushima S, Zhai P, Schneider MD, Sadoshima J (2010) NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc Natl Acad Sci U S A 107(35):15565–15570. doi:10.1073/pnas.1002178107
Kuroda J, Nakagawa K, Yamasaki T, Nakamura K, Takeya R, Kuribayashi F, Imajoh-Ohmi S, Igarashi K, Shibata Y, Sueishi K, Sumimoto H (2005) The superoxide-producing NAD(P)H oxidase Nox4 in the nucleus of human vascular endothelial cells. Genes Cells 10(12):1139–1151. doi:10.1111/j.1365-2443.2005.00907.x
Lagirand-Cantaloube J, Cornille K, Csibi A, Batonnet-Pichon S, Leibovitch MP, Leibovitch SA (2009) Inhibition of atrogin-1/MAFbx mediated MyoD proteolysis prevents skeletal muscle atrophy in vivo. PLoS One 4(3):e4973. doi:10.1371/journal.pone.0004973
Lee CF, Qiao M, Schroder K, Zhao Q, Asmis R (2010) Nox4 is a novel inducible source of reactive oxygen species in monocytes and macrophages and mediates oxidized low density lipoprotein-induced macrophage death. Circ Res 106(9):1489–1497. doi:10.1161/CIRCRESAHA.109.215392
Li J, Johnson SE (2006) ERK2 is required for efficient terminal differentiation of skeletal myoblasts. Biochem Biophys Res Commun 345(4):1425–1433
Londhe P, Davie JK (2011) Sequential association of myogenic regulatory factors and E proteins at muscle-specific genes. Skelet Muscle 1(1):14. doi:10.1186/2044-5040-1-14
Majmundar AJ, Skuli N, Mesquita RC, Kim MN, Yodh AG, Nguyen-McCarty M, Simon MC (2012) O(2) regulates skeletal muscle progenitor differentiation through phosphatidylinositol 3-kinase/AKT signaling. Mol Cell Biol 32(1):36–49. doi:10.1128/MCB.05857-11
Martin-Garrido A, Brown DI, Lyle AN, Dikalova A, Seidel-Rogol B, Lassegue B, San Martin A, Griendling KK (2011) NADPH oxidase 4 mediates TGF-beta-induced smooth muscle alpha-actin via p38MAPK and serum response factor. Free Radic Biol Med 50(2):354–362. doi:10.1016/j.freeradbiomed.2010.11.007
Mastroyiannopoulos NP, Nicolaou P, Anayasa M, Uney JB, Phylactou LA (2012) Down-regulation of myogenin can reverse terminal muscle cell differentiation. PLoS One 7(1):e29896. doi:10.1371/journal.pone.0029896
Megeney LA, Kablar B, Garrett K, Anderson JE, Rudnicki MA (1996) MyoD is required for myogenic stem cell function in adult skeletal muscle. Genes Dev 10(10):1173–1183
Mofarrahi M, Brandes RP, Gorlach A, Hanze J, Terada LS, Quinn MT, Mayaki D, Petrof B, Hussain SN (2008) Regulation of proliferation of skeletal muscle precursor cells by NADPH oxidase. Antioxid Redox Signal 10(3):559–574. doi:10.1089/ars.2007.1792
Montarras D, Lindon C, Pinset C, Domeyne P (2000) Cultured myf5 null and myoD null muscle precursor cells display distinct growth defects. Biol Cell 92(8–9):565–572
Moran JL, Li Y, Hill AA, Mounts WM, Miller CP (2002) Gene expression changes during mouse skeletal myoblast differentiation revealed by transcriptional profiling. Physiol Genom 10(2):103–111. doi:10.1152/physiolgenomics.00011.2002
Morgan MJ, Liu ZG (2011) Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res 21(1):103–115. doi:10.1038/cr.2010.178
Myer A, Olson EN, Klein WH (2001) MyoD cannot compensate for the absence of myogenin during skeletal muscle differentiation in murine embryonic stem cells. Dev Biol 229(2):340–350. doi:10.1006/dbio.2000.9985
Nishimura M, Nikawa T, Kawano Y, Nakayama M, Ikeda M (2008) Effects of dimethyl sulfoxide and dexamethasone on mRNA expression of housekeeping genes in cultures of C2C12 myotubes. Biochem Biophys Res Commun 367(3):603–608. doi:10.1016/j.bbrc.2008.01.006
Olguin HC, Olwin BB (2004) Pax-7 up-regulation inhibits myogenesis and cell cycle progression in satellite cells: a potential mechanism for self-renewal. Dev Biol 275(2):375–388. doi:10.1016/j.ydbio.2004.08.015
Pandey D, Fulton DJ (2011) Molecular regulation of NADPH oxidase 5 via the MAPK pathway. Am J Physiol Heart Circ Physiol 300(4):H1336–H1344. doi:10.1152/ajpheart.01163.2010
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29(9):e45
Piao YJ, Seo YH, Hong F, Kim JH, Kim YJ, Kang MH, Kim BS, Jo SA, Jo I, Jue DM, Kang I, Ha J, Kim SS (2005) Nox 2 stimulates muscle differentiation via NF-kappaB/iNOS pathway. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd = Retrieve&db = PubMed&dopt = Citation&list_uids = 15780757. Accessed 8/3/2012
Poppek D, Grune T (2006) Proteasomal defense of oxidative protein modifications. Antioxid Redox Signal 8(1–2):173–184. doi:10.1089/ars.2006.8.173
Potargowicz E, Szerszenowicz E, Staniszewska M, Nowak D (2005) [Mitochondria as an source of reactive oxygen species]. Postepy Hig Med Dosw (Online) 59:259–266
Powers SK, Duarte J, Kavazis AN, Talbert EE (2010) Reactive oxygen species are signalling molecules for skeletal muscle adaptation. Exp Physiol 95(1):1–9. doi:10.1113/expphysiol.2009.050526
Powers SK, Talbert EE, Adhihetty PJ (2011) Reactive oxygen and nitrogen species as intracellular signals in skeletal muscle. J Physiol 589(Pt 9):2129–2138. doi:10.1113/jphysiol.2010.201327
Schroder K, Wandzioch K, Helmcke I, Brandes RP (2009) Nox4 acts as a switch between differentiation and proliferation in preadipocytes. Arterioscler Thromb Vasc Biol 29(2):239–245. doi:10.1161/ATVBAHA.108.174219
Siu PM, Wang Y, Alway SE (2009) Apoptotic signaling induced by H2O2-mediated oxidative stress in differentiated C2C12 myotubes. Life Sci 84(13–14):468–481. doi:10.1016/j.lfs.2009.01.014
Sukhanov S, Semprun-Prieto L, Yoshida T, Michael Tabony A, Higashi Y, Galvez S, Delafontaine P (2011) Angiotensin II, oxidative stress and skeletal muscle wasting. Am J Med Sci 342(2):143–147. doi:10.1097/MAJ.0b013e318222e620
Tanaka K, Sato K, Yoshida T, Fukuda T, Hanamura K, Kojima N, Shirao T, Yanagawa T, Watanabe H (2011) Evidence for cell density affecting C2C12 myogenesis: possible regulation of myogenesis by cell–cell communication. Muscle Nerve 44(6):968–977. doi:10.1002/mus.22224
Wang HJ, Pan YX, Wang WZ, Zucker IH, Wang W (2009) NADPH oxidase-derived reactive oxygen species in skeletal muscle modulates the exercise pressor reflex. J Appl Physiol 107(2):450–459. doi:10.1152/japplphysiol.00262.2009
Xiao Q, Luo Z, Pepe AE, Margariti A, Zeng L, Xu Q (2009) Embryonic stem cell differentiation into smooth muscle cells is mediated by Nox4-produced H2O2. Am J Physiol Cell Physiol 296(4):C711–C723. doi:10.1152/ajpcell.00442.2008
Yoshida N, Yoshida S, Koishi K, Masuda K, Nabeshima Y (1998) Cell heterogeneity upon myogenic differentiation: down-regulation of MyoD and Myf-5 generates ‘reserve cells’. J Cell Sci 111(Pt 6):769–779
Yoshiko Y, Hirao K, Maeda N (2002) Differentiation in C(2)C(12) myoblasts depends on the expression of endogenous IGFs and not serum depletion. Am J Physiol Cell Physiol 283(4):C1278–C1286. doi:10.1152/ajpcell.00168.2002
Zammit PS, Relaix F, Nagata Y, Ruiz AP, Collins CA, Partridge TA, Beauchamp JR (2006) Pax7 and myogenic progression in skeletal muscle satellite cells. J Cell Sci 119(Pt 9):1824–1832. doi:10.1242/jcs.02908
Acknowledgments
This project was funded by USDA/NIFA award number 2010-34479-20715 “Increasing Shelf Life of Agricultural Commodities, ID.” The Optical Imaging Core at the University of Idaho has received support from the Idaho INBRE Program, NIH Grant Nos. P20 RR016454 (National Center for Research Resources) and P20 GM103408 (National Institute of General Medical Sciences), the M.J. Murdock Foundation, and the NIH-COBRE-Pathogenesis grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Acharya, S., Peters, A.M., Norton, A.S. et al. Change in Nox4 expression is accompanied by changes in myogenic marker expression in differentiating C2C12 myoblasts. Pflugers Arch - Eur J Physiol 465, 1181–1196 (2013). https://doi.org/10.1007/s00424-013-1241-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-013-1241-0