Abstract
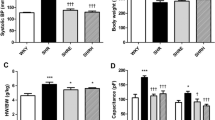
Changes in cellular calcium (Ca2+) handling are thought to underlie the altered contraction that occurs during cardiac hypertrophy and failure. Recent work has highlighted the importance of t-tubules in the control of intracellular Ca2+. The present study was performed to investigate whether changes in the distribution of I Ca between the surface and t-tubule membranes might contribute to the altered Ca2+ handling observed during compensated hypertrophy in the spontaneously hypertensive rat (SHR). Experiments were performed on ventricular myocytes isolated from 5-month-old SHR and normotensive Wistar-Kyoto (WKY) control rats. Osmotic shock using formamide was used to disrupt the t-tubular system and the whole-cell patch clamp technique used to monitor I Ca in the presence and absence of t-tubules. Membrane capacitance and I Ca were greater in control SHR than WKY myocytes; following detubulation, cell capacitance and I Ca both decreased and were no longer significantly different in the two cell types. The density of I Ca was not significantly different in control SHR and WKY cells or in detubulated myocytes from the two species. These data suggest that the distribution of I Ca is unchanged in SHR myocytes compared to WKY controls; I Ca density in the t-tubules was 1.2-fold greater than in the sarcolemma in both strains. These data also imply that the increase in surface area in SHR myocytes is due principally to an increase in t-tubular area, which is accompanied by an approximately equivalent increase in I Ca, so that the density of I Ca at the cell surface and in the t-tubules remains the same. These changes would be expected to retain cell function and synchronicity of Ca2+ release in the SHR at this stage of compensated hypertrophy.



Similar content being viewed by others
References
Balijepalli RC, Lokuta AJ, Maertz NA, Buck JM, Haworth RA, Valdivia HH, Kamp TJ (2003) Depletion of T-tubules and specific subcellular changes in sarcolemmal proteins in tachycardia-induced heart failure. Cardiovasc Res 59:67–77
Beuckelmann DJ, Wier WG (1988) Mechanism of release of calcium from sarcoplasmic reticulum of guinea-pig cardiac cells. J Physiol 405:233–255
Bouron A, Potreau D, Raymond G (1992) The L type calcium current in single hypertrophied cardiomyocytes isolated from the right ventricle of ferret heart. Cardiovasc Res 26:662–670
Brette F, Komukai K, Orchard CH (2002) Validation of formamide as a detubulation agent in isolated rat cardiac cells. Am J Physiol 283:H1720–H1728
Brette F, Salle L, Orchard CH (2004) Differential modulation of L-type Ca2+ current by SR Ca2+ release at the t-tubules and surface membrane of rat ventricular myocytes. Circ Res 95:e1–e7
Brette F, Salle L, Orchard CH (2006) Quantification of calcium entry at the t-tubules and surface membrane in rat ventricular myocytes. Biophys J 90:381–389
Brooksby P, Levi AJ, Jones JV (1993) Investigation of the mechanisms underlying the increased contraction of hypertrophied ventricular myocytes isolated from the spontaneously hypertensive rat. Cardiovasc Res 27:1268–1277
Brooksby P, Levi AJ, Jones JV (1992) Contractile properties of ventricular myocytes isolated from spontaneously hypertensive rat. J Hypertens 10:521–527
Cheng H, Cannell MB, Lederer WJ (1994) Propagation of excitation–contraction coupling into ventricular myocytes. Pflügers Arch 428:415–417
Delbridge LMD, Satoh H, Yuan W, Bassani JWM, Qi M, Ginsburg KS, Samarel AM, Bers DM (1999) Cardiac myocyte volume, Ca2+ fluxes, and sarcoplasmic reticulum loading in pressure-overload hypertrophy. Am J Physiol 272:H2425–H2435
Despa S, Brette F, Orchard CH, Bers DM (2003) Na/Ca exchange and Na/K-ATPase function are equally concentrated in transverse tubules of rat ventricular myocytes. Biophys J 85:3388–3396
Evans AM, Cannell MB (1997) The role of Ca2+ current and Na+ current-stimulated Na/Ca exchange in triggering SR calcium release in guinea-pig cardiac ventricular myocytes. Cardiovasc Res 35:294–302
Fabiato A (1985) Simulated calcium current can both cause calcium loading in and trigger calcium release from the sarcoplasmic reticulum of a skinned canine cardiac purkinje cell. J Gen Physiol 85:291–320
Fowler MR, Dobson RS, Orchard CH, Harrison SM (2004) Functional consequences of detubulation of isolated rat ventricular myocytes. Cardiovasc Res 62:529–537
Fowler MR, Naz JR, Graham MD, Bru-Mercier G, Harrison SM, Orchard CH (2005) Decreased Ca extrusion via Na/Ca exchange in epicardial left ventricular myocytes during compensated hypertrophy. Am J Physiol 288:H2431–2438
He JQ, Conklin MW, Foell JD, Wolff MR, Haworth RA, Coronado R, Kamp T (2001) Reduction in density of transverse tubules and L-type Ca2+ channels in canine tachycardia-induced heart failure. Cardiovasc Res 49:298–307
Kawai M, Hussain M, Orchard CH (1999) Excitation–contraction coupling in rat ventricular myocytes after formamide-induced detubulation. Am J Physiol 277:H603–H609
Keung EC (1989) Calcium current is increased in isolated adult myocytes from hypertrophied rat myocardium. Circ Res 64:753–763
Louch WE, Bito V, Heinzel FR, Macianskiene R, Vanhaecke J, Flameng W, Mubagwa K, Sipido KR (2004) Reduced synchrony of Ca2+ release with loss of t-tubules—a comparison to Ca2+ release in human failing cardiomyocytes. Cardiovasc Res 62:63–73
McCall E, Ginsburg KS, Bassani RA, Shannon TR, Qi M, Samarel AM, Bers DM (1998) Ca flux, contractility and excitation–contraction coupling in hypertrophic rat ventricular myocytes. Am J Physiol 274:H1348–H1360
McCrossan ZA, Billeter R, White E (2004) Transmural changes in size, contractile and electrical properties of SHR left ventricular myocytes during compensated hypertrophy. Cardiovasc Res 63:283–292
Nuss HB, Houser SR (1993) T-type Ca2+ current is expressed in hypertrophied adult feline left ventricular myocytes. Circ Res 73:777–782
Ryder KO, Bryant SM, Hart G (1993) Membrane current changes in left ventricular myocytes isolated from guinea pigs after abdominal aortic constriction. Cardiovasc Res 27:1278–1287
Shorofsky SR, Aggarwal R, Corretti M, Baffa JM, Strum JM, Al-Seikhan BA, Kobayashi YM, Jones LR, Wier WG, Balke CW (1999) Cellular mechanisms of altered contractility in the hypertrophied heart. Big hearts big sparks. Circ Res 84:424–434
Sipido KR, Volders PGA, Marieke de Groot SH, Verdonck F, Van de Werf F, Wellens HJJ, Vos MA (2000) Enhanced Ca2+ release and Na/Ca exchange activity in hypertrophied canine ventricular myocytes. Circulation 102:2137–2144
Volke T, Ehmke H (2002) Conservation of L-type Ca2+ current characteristics in endo- and epicardial myocytes from rat left ventricle with pressure-induced hypertrophy. Pflügers Arch 443:399–404
Acknowledgement
The authors gratefully acknowledge financial support from the British Heart Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fowler, M.R., Orchard, C.H. & Harrison, S.M. Cellular distribution of calcium current is unaltered during compensated hypertrophy in the spontaneously hypertensive rat. Pflugers Arch - Eur J Physiol 453, 463–469 (2007). https://doi.org/10.1007/s00424-006-0147-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-006-0147-5