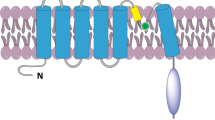
Abstract
Death of CNS neurons during acute injury occurs as a result of a complex combination of excitotoxicity, necrosis, apoptosis, oedema and inflammatory reactions. Neuroprotection via glutamate receptor blockade or antioxidant or anti-inflammatory therapy have not proven effective in the clinical treatment of brain damage due to narrow therapeutic windows, poor pharmacokinetics or blockade of the signalling essential for normal excitatory neurotransmission and neuronal survival. Recent work in neuronal biochemistry, genomics and proteomics has increased understanding of the molecular organization of the excitatory synapse and the neuronal postsynaptic density. Transient receptor potential (TRP) channels are an exciting new family of cation channels that are highly expressed in the brain. Several members can be induced by oxidative stress and oxygen free radicals, both of which play important roles in neurodegeneration. Recent work has indicated that members of the melastatin subfamily (TRPM) of TRP proteins, particularly TRPM7 and TRPM2, may play key roles in neuronal death that is activated by oxidative stress and downstream from excitotoxic signal pathways. This discovery provides an exiting new avenue for research into the pathophysiology and treatment of acute neurodegeneration.


Similar content being viewed by others
References
Olney JW (1969) Brain lesions, obesity, and other disturbances in mice treated with monosodium glutamate. Science 164:719–721
Choi DW (1988) Calcium-mediated neurotoxicity: relationship to specific channel types and role in ischemic damage. Trends Neurosci 11:465–469
Arundine M, Tymianski M (2003) Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium 34:325–337
Lipton P (1999) Ischemic cell death in brain neurons. Physiol Rev 79:1431–1568
Iadecola C (1997) Bright and dark sides of nitric oxide in ischemic brain injury. Trends Neurosci 20:132–139
Chan PH (1994) Oxygen radicals in focal cerebral ischemia. Brain Pathol 4:59–65
Dugan LL, Choi DW (1994) Excitotoxicity, free radicals, and cell membrane changes. Ann Neurol 35 (Suppl):S17–S21
Dawson VL, Kizushi VM, Huang PL, Snyder SH, Dawson TM (1996) Resistance to neurotoxicity in cortical cultures from neuronal nitric oxide synthase-deficient mice. J Neurosci 16:2479–2487
Birmingham K (2002) Future of neuroprotective drugs in doubt. Nat Med 8:5
Ikonomidou C, Turski L (2002) Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury? Lancet Neurology 1:383–386
Goldberg MP, Weiss JH, Pham PC, Choi DW (1987) N-methyl-D-aspartate receptors mediate hypoxic neuronal injury in cortical culture. J Pharmacol Exp Ther 243:784–791
Choi DW, Maulucci-Gedde M, Kriegstein AR (1987) Glutamate neurotoxicity in cortical cell culture. J Neurosci 7:357–368
Choi DW, Koh JY, Peters S (1988) Pharmacology of glutamate neurotoxicity in cortical cell culture: attenuation by NMDA antagonists. J Neurosci 8:185–196
Xiong Z, Lu W, MacDonald JF (1997) Extracellular calcium sensed by a novel cation channel in hippocampal neurons. Proc Natl Acad Sci USA 94:7012–7017
Xiong ZG, Chu XP, MacDonald JF (2001) Effect of lamotrigine on the Ca2+-sensing cation current in cultured hippocampal neurons. J Neurophysiol 86:2520–2526
Heinemann U, Lux HD, Gutnick MJ (1977) Extracellular free calcium and potassium during paroxysmal activity in the cerebral cortex of the cat. Exp Brain Res 27:237–243
Heinemann U, Pumain R (1980) Extracellular calcium activity changes in cat sensorimotor cortex induced by iontophoretic application of aminoacids. Exp Brain Res 40:247–250
Hansen AJ, Zeuthen T (1981) Extracellular ion concentrations during spreading depression and ischemia in the rat brain cortex. Acta Physiol Scand 113:437–445
Hille B (1992) Ionic channels of excitable membranes 2nd Edn. Sinauer, Sunderland
Clapham DE, Runnels LW, Strubing C (2001) The TRP ion channel family. Nat Rev Neurosci 2:387–396
Moran MM, Xu H, Clapham DE (2004) TRP ion channels in the nervous system. Curr Opin Neurobiol 14:362–369
Prawitt D, Monteilh-Zoller MK, Brixel L, Spangenberg C, Zabel B, Fleig A, Penner R (2003) TRPM5 is a transient Ca2+-activated cation channel responding to rapid changes in [Ca2+]i. Proc Natl Acad Sci USA 100:15166–15171
Wehage E, Eisfeld J, Heiner I, Jungling E, Zitt C, Luckhoff A (2002) Activation of the cation channel long transient receptor potential channel 2 (LTRPC2) by hydrogen peroxide. A splice variant reveals a mode of activation independent of ADP-ribose. J Biol Chem 277:23150–23156
Hara Y, Wakamori M, Ishii M, Maeno E, Nishida M, Yoshida T, Yamada H, Shimizu S, Mori E, Kudoh J, Shimizu N, Kurose H, Okada Y, Imoto K, Mori Y (2002) LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol Cell 9:163–173
Hardie RC, Raghu P (2001) Visual transduction in Drosophila. Nature 413:186–193
Agam K, von Campenhausen M, Levy S, Ben Ami HC, Cook B, Kirschfeld K, Minke B (2000) Metabolic stress reversibly activates the Drosophila light-sensitive channels TRP and TRPL in vivo. J Neurosci 20:5748–5755
Minke B, Agam K (2003) TRP gating is linked to the metabolic state and maintenance of the Drosophila photoreceptor cells. Cell Calcium 33:395–408
Hong YS, Park S, Geng C, Baek K, Bowman JD, Yoon J, Pak WL (2002) Single amino acid change in the fifth transmembrane segment of the TRP Ca2+ channel causes massive degeneration of photoreceptors. J Biol Chem 277:33884–33889
Yoon J, Ben Ami HC, Hong YS, Park S, Strong LL, Bowman J, Geng C, Baek K, Minke B, Pak WL (2000) Novel mechanism of massive photoreceptor degeneration caused by mutations in the trp gene of Drosophila. J Neurosci 20:649–659
Balzer M, Lintschinger B, Groschner K (1999) Evidence for a role of Trp proteins in the oxidative stress-induced membrane conductances of porcine aortic endothelial cells. Cardiovasc Res 42:543–549
Nadler MJ, Hermosura MC, Inabe K, Perraud AL, Zhu Q, Stokes AJ, Kurosaki T, Kinet JP, Penner R, Scharenberg AM, Fleig A (2001) LTRPC7 is a Mg.ATP-regulated divalent cation channel required for cell viability. Nature 411:590–595
Monteilh-Zoller MK, Hermosura MC, Nadler MJ, Scharenberg AM, Penner R, Fleig A (2003) TRPM7 provides an ion channel mechanism for cellular entry of trace metal ions. J Gen Physiol 121:49–60
Schmitz C, Perraud AL, Johnson CO, Inabe K, Smith MK, Penner R, Kurosaki T, Fleig A, Scharenberg AM (2003) Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell 114:191–200
He Y, Yao G, Savoia C, Touyz RM (2005) Transient receptor potential melastatin 7 ion channels regulate magnesium homeostasis in vascular smooth muscle cells: role of angiotensin II. Circ Res 96:207–215
Chubanov V, Waldegger S, Mederos y Schnitzler M, Vitzthum H, Sassen MC, Seyberth HW, Konrad M, Gudermann T (2004) Disruption of TRPM6/TRPM7 complex formation by a mutation in the TRPM6 gene causes hypomagnesemia with secondary hypocalcemia. Proc Natl Acad Sci USA 101:2894–2899
Hanano T, Hara Y, Shi J, Morita H, Umebayashi C, Mori E, Sumimoto H, Ito Y, Mori Y, Inoue R (2004) Involvement of TRPM7 in cell growth as a spontaneously activated Ca2+ entry pathway in human retinoblastoma cells. J Pharmacol Sci 95:403–419
Schmitz C, Perraud AL, Fleig A, Scharenberg AM (2004) Dual-function ion channel/protein kinases: novel components of vertebrate magnesium regulatory mechanisms. Pediatr Res 55:734–737
Takezawa R, Schmitz C, Demeuse P, Scharenberg AM, Penner R, Fleig A (2004) Receptor-mediated regulation of the TRPM7 channel through its endogenous protein kinase domain. Proc Natl Acad Sci USA 101:6009–6014
Ryazanova LV, Dorovkov MV, Ansari A, Ryazanov AG (2004) Characterization of the protein kinase activity of TRPM7/ChaK1, a protein kinase fused to the transient receptor potential ion channel. J Biol Chem 279:3708–3716
Dorovkov MV, Ryazanov AG (2004) Phosphorylation of annexin I by TRPM7 channel-kinase. J Biol Chem 279:50643–50646
Aarts M, Iihara K, Wei WL, Xiong ZG, Arundine M, Cerwinski W, MacDonald JF, Tymianski M (2003) A key role for TRPM7 channels in anoxic neuronal death. Cell 115:863–877
Feighan D, MacVicar BA (2004) A Gadolinium-sensitive cation channel contributes to ischemic-like excitotoxicity in brain slices in addition to NMDA and kainate receptor activation. 2004 Abstract Viewer/Itinerary Planner Washington DC: Society for Neuroscience 99.16 http://sfn.scholarone.com/itin2004/index.html
Manev H, Favaron M, Guidotti A, Costa E (1989) Delayed increase of Ca2+ influx elicited by glutamate: role in neuronal death. Mol Pharmacol 36:106–112
Chinopoulos C, Tretter L, Rozsa A, Adam-Vizi V (2000) Exacerbated responses to oxidative stress by an Na+ load in isolated nerve terminals: the role of ATP depletion and rise of [Ca2+]i. J Neurosci 20:2094–2103
Rego AC, Ward MW, Nicholls DG (2001) Mitochondria control AMPA/kainate receptor-induced cytoplasmic calcium deregulation in rat cerebellar granule cells. J Neurosci 21:1893–1901
Hartley DM, Choi DW (1989) Delayed rescue of N-methyl-D-aspartate receptor-mediated neuronal injury in cortical culture. J Pharmacol Exp Ther 250:752–758
Tymianski M, Charlton MP, Carlen PL, Tator CH (1993) Secondary Ca2+ overload indicates early neuronal injury which precedes staining with viability indicators. Brain Res 607:319–323
Limbrick DD Jr, Pal S, DeLorenzo RJ (2001) Hippocampal neurons exhibit both persistent Ca2+ influx and impairment of Ca2+ sequestration/extrusion mechanisms following excitotoxic glutamate exposure. Brain Res 894:56–67
Chen QX, Perkins KL, Choi DW, Wong RK (1997) Secondary activation of a cation conductance is responsible for NMDA toxicity in acutely isolated hippocampal neurons. J Neurosci 17:4032–4036
Chinopoulos C, Gerencser AA, Doczi J, Fiskum G, Adam-Vizi V (2004) Inhibition of glutamate-induced delayed calcium deregulation by 2-APB and La3+ in cultured cortical neurones. J Neurochem 91:471–483
Rowland KC, Connor JA, Shuttleworth CWR (2004) Mechanisms underlying the initiation of sustained inward currents in CA1 hippocampal neurons. 2004 Abstract Viewer/Itinerary Planner Washington, DC: Society for Neuroscience, 2004 Online 342.4. http://sfn.scholarone.com/itin2004/index.html
Jiang X, Newell EW, Schlichter LC (2003) Regulation of a TRPM7-like current in rat brain microglia. J Biol Chem 278:42867–42876
Runnels LW, Yue L, Clapham DE (2002) The TRPM7 channel is inactivated by PIP2 hydrolysis. Nat Cell Biol 4:329–336
Choi DW, Koh JY (1998) Zinc and brain injury. Annu Rev Neurosci 21: 347–375
Harteneck C, Kuchta SN, Huber A, Paulsen R, Schultz G (2002) The PDZ scaffold protein INAD abolishes apparent store-dependent regulation of the light-activated cation channel TRP. FASEB J 16:1668–1670
Li HS, Montell C (2000) TRP and the PDZ protein, INAD, form the core complex required for retention of the signalplex in Drosophila photoreceptor cells. J Cell Biol 150:1411–1422
Tang Y, Tang J, Chen Z, Trost C, Flockerzi V, Li M, Ramesh V, Zhu MX (2000) Association of mammalian trp4 and phospholipase C isozymes with a PDZ domain-containing protein, NHERF. J Biol Chem 275:37559–37564
Mery L, Strauss B, Dufour JF, Krause KH, Hoth M (2002) The PDZ-interacting domain of TRPC4 controls its localization and surface expression in HEK293 cells. J Cell Sci 115:3497–3508
Shieh BH, Zhu MY (1996) Regulation of the TRP Ca2+ channel by INAD in Drosophila photoreceptors. Neuron 16:991–998
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aarts, M.M., Tymianski, M. TRPMs and neuronal cell death. Pflugers Arch - Eur J Physiol 451, 243–249 (2005). https://doi.org/10.1007/s00424-005-1439-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-005-1439-x